KSEEB Class 9 Biology Chapter 5 Natural Resources Notes Learning Objectives
- After completing this chapter, you will be able to
- define natural resources and recognize various zones of earth;
- differentiate between abiotic and biotic components of biosphere;
- recognize air as breath of life;
- explain how wind is caused and how it causes rains;
- describe various sources, effects, prevention and control of air and water pollution;
- list various processes and factors that make soil;
- understand the causes and prevention of soil pollution and soil erosion;
- describe biogeochemical cycles of water, nitrogen, carbon and oxygen;
- explain the relationship between greenhouse effect and global warming;
- describe various causes of ozone layer depletion.
‘‘Earth is the only planet which has all essential conditions required for die existence and survival of life. Life on the earth depends on many (actors, such as an optimum temperature, abundance of water and food and air to breath. These resources and the energy obtained from the sun are necessary for meeting the basic requirements of all life forms present on the earth.”
Natural Resources And The Biospherre
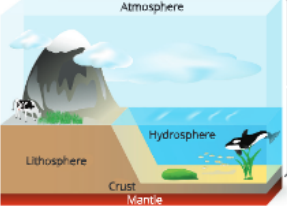
- All kinds of material required by man to meet his various basic requirements for survival and continuance are known as resources. These are obtained from nature, thus, they arc known as natural resources. These natural resources are soil (land), wrater, air, etc. These resources are present in mainly three zones, namely, lithosphere, hydrosphere and atmosphere.
- Lithosphere The outer crust of the earth is called lithosphere. Its upper weathered thin layer is called soil.
- Hydrosphere The zone in which water exists is known as hydrosphere. Water covers about 75 per cent of the earths surface and occurs in oceans, river, lakes, ponds, dams, etc. It is also found in underground water resources.
- Atmosphere The zone w’here the air covers the earth like a blanket is called atmosphere. Living beings are found only where all these tliree zones exist.

Biosphere
- The life-supporting zone of rhe earth where all rhese three zones, namely, atmosphere, hydrosphere and lithosphere interact with each other, making life possible is called biosphere.
Components of biosphere – Biotic and abiotic
- There arc two components of biosphere – biotic and abiotic. The biotic components of biosphere are the living beings. Various microorganisms, plants and animals form the biotic components of biosphere.
- Those components of biosphere that do not have life are called abiotic or non-living components. Air, water, soil, light and temperature form the non-living or abiotic components of biosphere.
- Some abiotic components of atmosphere are discussed in this chapter in order to understand their role in sustaining life on the earth.
Air – The Breath Of Life
- Air (which forms die atmosphere) is one of the most important elements of our physical surroundings. It is essential for the survival of all living organisms.
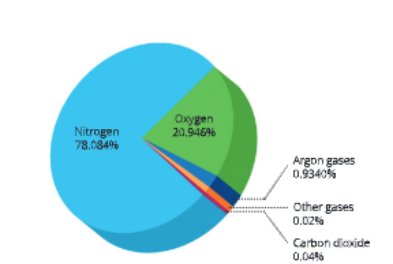
- Air is an important inexhaustible natural resource. It is a mixture of gases like oxygen (02), nitrogen (N2), carbon dioxide (C02), ammonia (NH3), argon, helium, ozone and water vapour.
- The composition of air is the basis of life on the earth. Oxygen is not abundant on planets where life does not exist On planets such as Venus and Mars (where there is no life), the major component of the atmosphere is carbon dioxide, which constitutes up to 95-97 per cent of their atmosphere.
- The composition of air remains almost the same because its consumption is counter balanced with Its production. In die earlier chapters, you have studied that all eukaryotic and many prokaryotic cells need oxygen for respiration during which oxygen is used to break down glucose molecules to get energy for various life processes. Burning of fuels and forest fires also utilize oxygen and produce carbon dioxide. Thus, both during respiration and combustion, oxygen is used and carbon dioxide is produced.
- However, even after this, the percentage of carbon dioxide in our atmosphere does not change and remains almost the same. It remains merely a fraction of a per cent (about 0.03-0.04%) because carbon dioxide is fixed in two ways.
- Green plants use carbon dioxide and water, and convert these into glucose (carbohydrate) in the presence of sunlight during the process of photosynthesis.
- Many marine animals like molluscs use carbonates dissolved in sea water to make their body shells. This fixes the C02 dissolved in water as carbonates.
Natural Resources Chapter Summary Class 9
Importance of atmosphere (air)
- Oxygen in the air is necessary for respiration. Without oxygen, all living beings will die. In nature, it is also dissolved in water. Oxygen keeps the water fresh and it is also a source of respiration for aquatic life.
- Oxygen is a supporter of combustion. Without oxygen, combustion cannot occur.
- Oxygen combines with almost all elements to form oxides.
- Air is also necessary for controlling atmospheric temperature.
- Nitrogen in the air is required by plants to manufacture proteins. All living beings obtain proteins from plants directly or indirectly.
Activity-1
- To study the variation in temperature of various media and compare it with the temperature of the atmosphere
You will need - Glass beakers, a bottle, soil/sand, water, thermometer
Procedure
- Take two beakers. Fill one beaker with water and the other beaker with soil/sand. Label them as A and B, respectively.
- Take a closed bottle containing thermometer. Label
- Keep all these vessels in bright sunlight for three hours.
- After three hours, measure the temperature of all the three vessels. Also take the temperature reading of air in the shade at the same time
What do you observe in the above mentioned activity?
Observation
- Is die temperature reading higher in beaker A or B?
- Based on the above findings, which of die following would heat faster the land or the sea?
- Is the thermometer reading of temperature of air in the shade same as that of sand or water? What is the reason for this?
- Is die temperature of air in die closed glass botde the same as that of die open air? Wliat is the reason for this? Have you ever come across such a phenomenon in day to day life?
Inference
- Temperature reading is higher in beaker B.
- The land wili get heated faster.
- No, the temperature of air in the shade is less than that of sand or water.
- No, the temperature is higher in the closed glass bottle than diat of the open air. This is because the heat is trapped in the glass botde. Such a phenomenon is seen in the greenhouse.
Conclusion
- The observations in the above activity reveal that sand and water do not heat up at the same rate.
- What do you think will be their rates of cooling?
- Carbon dioxide is an important component of air.
- Plants need carbon dioxide for producing food through photosynthesis.
- Carbon dioxide is used in fire extinguishers.
- Air movement causes winds and rains.
KSEEB Class 9 Biology Important Questions Chapter 5
Role Of The Atmosphere In Climate Control
- In the previous section, you have read that atmosphere covers die earth like a blanket. You know that air is a bad conductor of heat What is die role of atmosphere in controlling the climate? The atmosphere keeps die average temperature of the eardi steady during daytime and also during die whole year.
- It prevents the sudden increase in temperature during daytime. It also slows down die escape of heat into outer space during night and as a result does not let the weather become too cold during night.
- However, reverse happens on die moon which does not have atmosphere. Although the moon is at almost the same distance away from the sun as the earth, it shows great variation in temperature during daytime and night Hie surface temperature of the moon ranges from -190 °C (during night) to 110 “C (during the day) since there is no atmosphere on the moon. Because of so much variation in temperature, there is no life on the moon.
The Movement O Air – Wind
- You would have observed that many a times, after a hot day, there is cool breeze in the evening. Sometimes, it rains after few days of really hot weather. How does this happen?
- What causes the movement of air or rainfall?
- What makes this movement of air a gende breeze, a strong wind or storm? All diese phenomena are the result of changes that take place in the atmosphere due to the heating of air and the formation of water vapour.
- But you may wonder as to how is water vapour formed?
- How is water vapour formed?
- Water vapour is formed due to the heating ofwatertnxiies like oceans, lakes, rivers and ponds by the suns rays as well as due to the activities of living organisms.
- The atmosphere is heated by the radiation that is reflected back or re-radiated by the land surface or by the waterbodies.
- As a result ot being heated up by the radiation, convection currents are set up in the air and water vapour is formed. Let us perform an activity to understand the nature of convection currents.
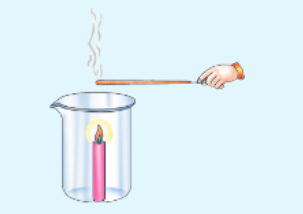
Activity-2
To study the nature of convection currents You Hill need
Candle, glass beaker or a unde-mouthed bottle, incense sticks, matchbox
Procedure
Take a glass beaker or unde-mouthed botde and fix a candle in it Light the candle, o Take an incense stick and light it Take this incense stick near the mouth of the beaker and observe.
Observations
What do you observe in the above-mentioned activity?
Observation
- In which direction docs die smoke flow when the Incense stick is kept near die edge of the mouth of the beaker?
- In which direction does die smoke flow when the incense stick is kept a little above the candle?
- In which direction does the smoke flow when the incense stick is kept at other places around beaker?
Inference
- The smoke moves towards the flame.
- The smoke rises up.
- The smoke moves towards the beaker.
Conclusion
- The observations on the smoke in the above activity reveals the directions in which hot and cold air move. 1 he smoke moves towards the low pressure area.
How is wind caused?
- Wind is caused by the differences in the atmospheric pressure between two or more places. Air from the area of high pressure moves towards die area of lower pressure. This movement of air causes wind or breeze.
- When sun rays fall on the earth, die air gets heated up by radiation and from the heated land or water, it rises up. As the land and water get heated up at different rates, convection currents are set in. Since die land gets heated up faster than water, the air over the land would be heated up faster than the air over waterbodies.
- In coastal regions that are closer to sea, the air above the land gets heated up faster and starts rising up during the day. Hius, a region of low pressure is created over land and air from the sea moves into this area of low pressure over land. This movement of air from one region to die odier causes winds.
What is the direction of wind during day and night?
- During day ’The direction of the wind is from the sea to the land.
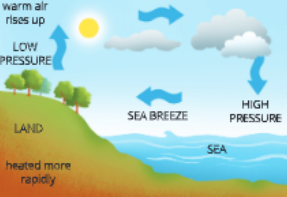
- During night Both land and sea start cooling down. However, since water cools down slower
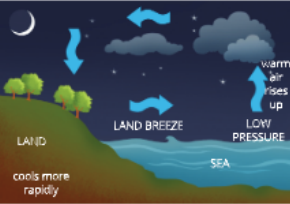
- Than the land, the air above water would be wanner than tiie air above land. Hence, the direction of the wind is from the land to the sea.
How does air move in diverse directions?
- Many a times, you would have observed that air moves in different directions. This phenomena of movement of air in diverse directions is caused due to
- the uneven heating of land in different regions of the earth,
- the rotarion of the earth,difference in the rates of vaporization and condensation of water vapour, and
- the presence of mountain ranges in the paths of wind, which disrupt the easy flow of air.
How Is Rain Caused?
- Let us perform an activity to understand how clouds are formed and what are the various factors that influence climate change.
Activity-3
- To demonstrate various factors influencing climatic changes
Yon will need - An empty plastic bottle, water, incense stick and matchbox Procedure
- Take an empty plastic water battle.
- Pour about 5 10 rnL of water into it and close die bottle rightly. Shake it well or leave it our in the sun for 10-15 minutes. ‘Ihis causes the air in the bottle ro be saturated with water vapour.
- Take an incense stick and light it. Open the cap of the bottle and allow some smoke from the incense stick to enter the bottle. Quickly dose the bottle once again. Make sure that the cap fits tightly.
- Press the botdc hard between your hands and try to crush it as much as possible. Wait lor a few seconds and release it.
- Now, again press the bottle as hard as you can and rry to crush it.
KSEEB SSLC Chapter 5 Notes Detailed Explanation
Observations
What, do you observe in the above mentioned activity? Answer the following questions and complete the table. One is done for you.
Inference
Observation
Did che air inside seem co become foggy? When did you observe this?
When did this fog disappear?
When was the pressure inside the bottle high?
Was the fog observed in die bottle when the pressure was high or observed when it was low?
Why were the smoke panicles introduced inside the bottle for this experiment?
What will happen if this experiment is performed without the smoke from live incense stick?
Inference
Yes, on introduction of smoke from incense stick, the air inside became foggy.
Conclusion
The observations in the above activity show what happens when air with a very high content of water vapour moves from a region of high pressure to a region of low pressure or vice versa.
- In Activity 3, you learnt what happens when air with a very high content of water vapour moves from a region of high pressure to a region of low pressure or vice versa.
- The process of formation of rain is given below.
- During daytime, due to the energy of the sun, waterbodies are heated and a large amount of water evaporates and goes into die air.
- Some of the water vapour also gets into the atmosphere because of various biological activities,At the same time, air also gets heated up. It rises up and carries the water vapour with it.
- As the air rises up, it expands and cools down. This cooling causes the condensation of water vapour in the air into tiny droplets.
- Tliis condensation of water is facilitated by some particles of dust that act as the nucleus’ around which these drops are formed. Normally dust and other invisible particles are suspended in the air which help in the formation of droplets of water.
- The water droplets condense further and grow bigger in size. Soon the droplets grow so big and heavy that they can no longer remain suspended in the air and fall down in the form of rain drops. Sometimes, when the temperature of air is very low, water may precipitate in the form of snow, sleet or hail.
- How are rainfall patterns decided?
- You must have seen that at some places, the rainfall is very high while at other places, it is very low. Many a times, this difference is seen within a small area. How docs this happen? What is the reason for such a pattern of rainfall?
- Rainfall patterns arc decided by the prevailing wind patterns. In large parts of India, rains are mostly brought about by the south-west or north¬east monsoons. Sometimes you
- would have heard the weather reports stating that ‘depression’ in the Bay of Bengal has caused rains in some areas. Monsoon winds are seasonal and associated with rainfall. You will learn 116 more about rains in higher classes.
Activity-4
- To find out about the rainfall patterns in your area You will need
- Newspaper clippings or weather reports on television, rain gauge.
Procedure
- Collect information from newspapers or from weather reports on the television about rainfall patterns across the country.
- Construct a rain gauge and record observations. To construct a rain gauge, take a measuring cylinder and put some oil into it. This will prevent the evaporation of the rainwater that will fall in it. Put it in a container. Fix a funnel on its mouth and cover it with a porous cap as

- Take aU the precautions to get reliable data from this rain gauge.
- Now answer the following questions
- In which monrk did your ciry/town/village ger the maximum rainfall?
- In which month did your region.’state get the maximum rainfall?
- Whether rain was always accompanied by thunderstorm and lightning? If not, then which was the season when you got more of thunder and lightning with the rain?
Activity-5
To find out more about monsoons and the rainfall patterns in India and compare the findings with other countries
Go to your school library and find out more about monsoons and cyclones.
IV)’ and find out the rainfall pattern of India and compare it with other countries.
Is monsoon responsible for rains all over the world
Air Pollution
- Clean and pure air is very essential for the health and survival of man. However, with the progress in man’s living standards, air has become more polluted.
- Pollution can be defined as an undesirable change in the physical, chemical and biological characteristics of our surroundings which harms human life and other living beings. The substances that cause such changes, Le. pollution, are called pollutants.
- Air pollution can be defined as the occurrence or addition of foreign particles, gases and other materials into the air, which adversely affect the health of living organisms, vegetation, buildings and monuments.
- Industrial wastes and automobile exhausts arc the two major sources of various air pollutant

Sources of air pollution
The substances that cause air pollution are known as air pollutants. Some of the major air pollutants are given .
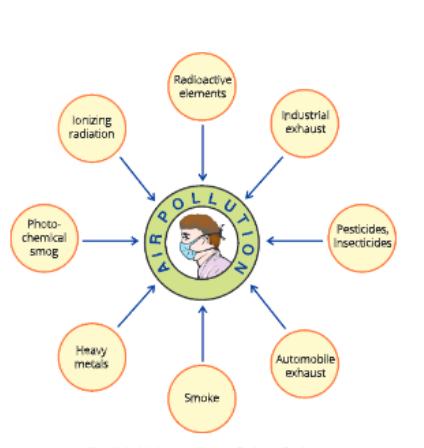
Carbon dioxide
Carbon dioxide is one of the gases present in the atmosphere and is used by plants for photosynthesis. It is chiefly produced during the combustion of fuels in households, factories, power stations, etc. The level of carbon dioxide has increased over the period of time. Carbon dioxide is injurious to health and it may lead to a rise in the atmospheric temperature due to greenhouse effect.
Natural Resources Class 9 KSEEB Question Answers
Monoxide
Carbon monoxide is produced as a result of incomplete combustion of wood, charcoal and fossil fuels like coal and petroleum. Automobiles using diesel and petroleum are the major sources of carbon monoxide.
Carbon monoxide is more dangerous than carbon dioxide. It is a poisonous gas that leads to respiratory problems. It also causes giddiness, headache and cardiovascular malfunctioning.
Oxides of nitrogen and sulphur
- These are produced by the burning of fossil fuels like coal and petroleum in powerhouses and automobiles, respectively. These fuels contain small amounts of nitrogen and sulphur. When these fuels are burnt, diff erent oxides of nitrogen and sulphur are produced.
- The oxides of nitrogen and sulphur combine with water to form nitric acid and sulphuric acid, respectively.
- These acids dissolve in rainwater and tall as acid rain.
- Acid rain causes lot of damage to monuments and buildings as well as vegetation. If inhaled, these oxides cause irritation to the eyes and respiratory diseases like asthma and bronchitis.
Smog
- Smog is a mixture of smoke, dust particles and small drops of fog formed due to the condensation of water. The combustion of fossil fuels increases the amount of suspended particles in air. These particles maybe either unbumt carbon particles or hydrocarbons Presence of high levels of suspended particulate matter and other pollutants may cause lower visibility, especially in cold weather. Smog may cause necrosis and develop a white coating on the leaves of plants. In human beings and animals, it may cause asthma and allergies.
Effects of air pollution on human health
- Carbon monoxide combines with haemoglobin molecules in human blood and causes suffocation. Depletion of ozone layer due to chlorofluorocarbons (CFCs) causes skin cancer as a result of overexposure of the human skin to ultraviolet rays. Sulphur-dioxide-originated smog blocks the human, respiratory system, which leads to death. Sulphur dioxide also causes diseases of the eyes, throat, nose and lung infections. It also causes acid rain.
- Nitric oxide (NO) in high concentration causes respiratory’ problems, internal bleeding, oxygen deficiency, pneumonia and lung cancer.
- Air pollutants like suspended particulate matter (SPM) cause asthma, lung cancer and asbestosis SPM are small sized (particulate) air pollutants, which remain suspended in air for a very long time Smoke, dust, unburnt carbon particles (soot), fly ash, etc. form SPM.
- Air pollution reduces soil moisture and thus agricultural crops are damaged, resulting in heavy economic losses to farmers.
Prevention and control of air pollution
There are two types of air pollutants – Gaseous Particulate
Methods of controlling gaseous pollutants
Combustion In this technique, organic pollutants are converted into less harmful products such as C02 and water vapour.
Absorption In this technique, gaseous pollutants are passed through absorbing materials like scrubbers. This absorbent removes pollutants present in the gaseous emission.
Adsorption Adsorption is a process in which a substance sticks to the surface of another substance (called adsorbent), hi this technique, gaseous emissions are passed dirough porous solid adsorbents kept in suitable containers. The gaseous pollutants stick or are adsorbed on the surface of the porous material and clean air passes through.
Methods of controlling particulate air pollutants
The particulate air pollutants such as dust, soot and fly ash can be controlled by using fabric filters, electrostatic precipitators, wet scrubbers and mechanical devices. These are described here.
Fabric filters hi diis technique, gaseous emission containing dust, soot and fly ash is passed through porous fabric filters made of woven or filled fabric. The particles of pollutants present in the gas get trapped in this fabric and arc collected in the filter and the gases free from the pollutant particles are discharged.
Wet scrubbers Wet scrubbers are used in chemical, metallurgical and mining industries. The wet scrubbers trap sulphur dioxide, ammonia and metal fumes in their tank, discharging clean gases into the atmosphere.
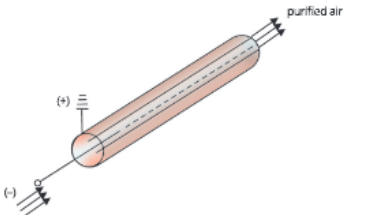
Electrostatic precipitators In this technique, a gas or air steam containing aerosols in the form of dust, mist or fumes is passed between the two electrodes of an electrostatic precipitator. During this process, tire aerosol particles get precipitated on the electrode (Fig. 5.9).
In addition to the above, air pollution may also be prevented and controlled by the following measures By using better-designed equipment and smokeless fuels/hearths in industries and at home.
By relocating industries causing pollution to remote areas (thereby diluting the pollution), v Using precipitators, scrubbers and filters to control particulate matter produced by industries.
Using environment-friendly fuels, such as compressed natural gas (CNG) in automobiles instead of petrol/diesel.
Planting more and more trees surrounding industrial establishments and along the roadside to reduce C02 level in the environment Creating awareness in public through films, lectures, street plays and debates about the harmful effects of air pollution.
Activity-6
To study the effect ot’ pollutants on lichens growing on the bark of trees
You have learned about lichens. They are very sensitive to the levels of contaminants like sulphur dioxide in the air. They can be easily found growing on the barks of trees as a thin greenish-white crust

Procedure
Compare the lichens on trees near busy roads and trees away from roads.
Observe the trees near roads, and compare the lichens on the side facing the road and on the side away from the road.
Observation
What do you observe? What can you say about the levels of polluting substances near roads and away from roads on the basis of your findings above?
Inference
The growth of lichens is more in the trees found away from the road. Also, lichens are found more on the trees facing away from the road.
Conclusion
From the above activity, you can conclude that pollutants affect the growth of lichens in a given area.
Class 9 Biology Chapter 5 Natural Resources Types And Uses
Water
Is the availability of water same everywhere?
Almost two-third of the earths surface contains water. It is also found underground. Some amount of water is also found in the form of water vapour in the atmosphere. Sea and oceans contain most of the water available on the earth’s surface. However, this water is saline. Freshwater is found frozen in the form of ice caps on the two poles (North Pole and South Pole) and on snow-covcrcd mountains. Freshwater is also available in rivers, lakes and ponds. Underground water is also fresh. However, the availability of freshwater is not same everywhere. It varies from place to place. At some places, it is available in enough quantity while at most other places, there is shortage of water. At times, people living in rural areas have to travel long distances to fetch water for drinking and domestic use. Over a period of time, the level of groundwater has dcplercd at most places.
Activity-7
To find about water harvesting techniques -Find out about the water harvesting techniques to improve the availability of water in your area.
Find out how they increase the availability of water for use.
Why is water necessary?
Water is necessary to carry out various life activities. Existence of life is not possible without water. It is the prime constituent of all living cells. All cellular processes take place in aqueous medium in our body In our body, various substances arc found dissolved in water which react within the cells or within the body. Water is also needed for transportation of various substances such as nutrients from one part of our body to another. Thus, it is necessary for organisms to maintain a certain level of water in their body for survival.
Terrestrial animals and plants require fresh water because saline water contains high amounts of salts and their bodies cannot tolerate or get rid of this high amounts of salts in saline water.
Thus, the water is necessary for the survival of plants and animals on the earth. Besides being a basic human need, it is also ait important and precious national asset.
Activity-8
To find out the effect of different climates on the occurrence of biodiversity in a given area Procedure
Go out in open and select a small area (say. 2 m2) in some unused land in or near a river,stream,lake,pound.
-Count the number of different organisms and plants in dtis area. You may not find any larger animals but you can easily locate organisms such as insects, earthworm and ants. Also, check the number of individuals of each type or species.
Select another area of same dimension near a rock)’ region. Compare the number of individuals (both animals and plants) found in this area with those observed in the area selected near a waterbody.
Observations
What do you observe? Is the variety of plant and animal life same in both these areas?
ACTIVITY-9
To find out the effect of different climates on the occurrence of biodiversity in a given area Procedure
Go out in open and select a small area (say. 2 m2) in some unused land in or near your school.
Counr the number of different organisms and plants in this area. Check the number of individuals of each type or species.
Take your observations twice a year – once during dry season and second during rainy season.
Compare the number of individuals (both animals and plants) found in this area at both the instances.
Observations What do you observe?
Inference
Observation
Whether the numbers were similar both the times?
In which season did you find more variety of plants and animals?
In which season were the number of individuals of each variety more?
Inference
No. Since the environmental conditions were different each time.
In rainy season, there was more variety of plants and animals.
In rainy season, the number of individuals of each variety was more.
From the two activities (Activities 8 and 9), you will conclude that there is a relationship between the amount of water available and the quantity (number) and variety of plants and animals found in that area. You will find a greater variety and abundance of life in a region which receives greater (for example 200 cm) rainfall in a year. Hi us, states like Kerala and Puducherry which receive greater rainfall have the maximum biodiversity. On the other hand, states like Rajasthan, which receive lesser rainfall have least biodiversity.
Water is one of the major natural resources, which determines the availability of life on land. The availability of water not only decides tire number of individuals of each species that can survive in a particular area but also the diversity of life in that area. In addition, there are other factors such as temperature and light, which also determine the sustainability of life in a region.
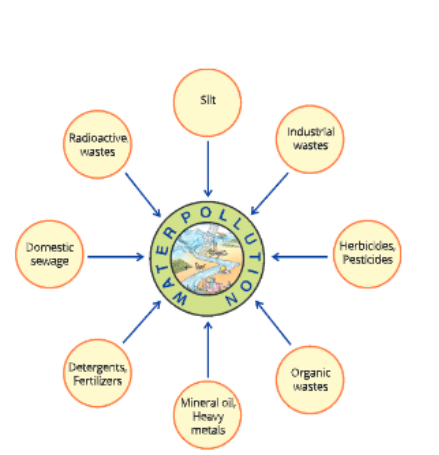
Water Pollution
Water pollution can be defined as a change in the physical, chemical and biological properties of water by the addition of undesirable substances or the removal of desirable substances from waterbodies or a change in temperature of water, which may have harmful effects on human and aquatic life.
Sources of water pollution
Water gets polluted in many ways
Fertilizers and pesticides Fertilizers and pesticides are used in forms to get higher yields of food crops. These fertilizers and pesticides dissolve in water and are washed into waterbodies like lakes and rivers and seep into groundwater.
Domestic sewage The sewage from our houses is discharged into rivers and lakes.
Industrial wastes Our industries produce a lot of waste containing high concentration of oil, heavy metals and detergents. This waste is dumped into rivers or lakes. There are many industries which use water in various operations for cooling and later return this hot water to waterbodies, which raises their temperature.

All these activities can affect the life forms found in waterbodies. These can encourage the growth of some life forms while affecting the growth of some other life forms available there. This causes an imbalance between various organisms living there.Thus, the term water pollution can be used to cover the following aspects
Addition of undesirable substances to water-bodies, like
fertilizers and pesticides used in farming; or
poisonous substances, such as mercury salts which are used in paper industries to manufacture paper; or
disease-causing microorganisms such as bacteria that causes cholera.
All these substances when added to water adversely affect die aquatic life. The toxic materials in water may enter the food chain and cause serious health hazards to human beings and odier aquatic animals. Epidemics, such as cholera, jaundice, dysentery and diarrhoea may spread.
Removal of desirable substances from water¬bodies Oxygen is also found dissolved in water.
Dissolved oxygen in water is used by the animals and plants that live in water. Any change in water could reduce the amount of dissolved oxygen and adversely affect these aquatic organisms. This could also lead to the depletion of other nutrients from the waterbodies.
Change in temperature Aquatic organisms can survive well up to a certain range of temperature. A sudden change in this temperature maybe dangerous for these organisms or aflect their breeding. The eggs and larvae of various organisms are particularly susceptible to temperature changes, which cannot survive a drastic change in temperature.
Prevention and control of water pollution
The control of water pollution requires many remedial measures involving individuals, cuiniuuuily and governments. Some steps that may reduce water pollution are
Setting up sewage water treatment plants.
Using septic tanks in houses to avoid direct dumping of faecal matter and other wastes.
Avoiding contamination of rivers, lakes and ponds by washing clothes, bathing, etc.
Not throwing waste food materials, paper, biodegradable vegetables and plastic into open drains.
Treating industrial cfllucnrs before discharging into rivers, making separate channels for river and sewage water.
Generating public awareness about tire maintenance of ponds, river, lakes and wells in rural and urban areas.
KSEEB SSLC Natural Resources Short Notes Class 9
Soil And Minerls
The earth we live on is covered with soil. Soil is one of the important resources that influence and decide the diversity of life in an area.
The outermost layer of the earth is known as crust The minerals found in the earths crust supply many nutrients to living beings. These minerals arc usually bound in huge rocks. Over long periods of time (thousands and millions of years), the rocks on the surface of the earth are broken down by various physical, chemical and biological processes. This breaking down gives us fine particles as end products known as soil.
Soil formation – Pedogenesis
The process of breaking down of huge pieces of rocks and its minerals into fine particles due to continuous action of physical, chemical and biological agents is called weathering. Depending on the type of natural agent involved, weathering can be classified as physical, biological and chemical weathering.
Physical weathering
It is the weathering of rocks by variation in temperature, water and wind.
The Sun The sun plays an important role in soil formation. During daytime, rocks get heated up due to suns energy. As a result, they expand. At night, when tire temperature lowers down, these rocks cool down and contract. However, all parts of rocks do not expand or contract at the same rate. As a result of difference in the rate of contraction in various parts of rocks, cracks are formed in the rocks. Ultimately, huge rocks break down into smaller pieces of soil particles.
Water Water also plays an important role in the formation of soil in two ways
It gets into the cracks in the rocks which are formed due to the uneven heating by the sun. Later when this water freezes, it causes the cracks to widen.
Water flowing over the rocks during long periods of time wears away even hard rock. Fast flowing water often carries big and small particles of rock downstream. During water flow, these rocks liit other rocks and the resultant collisions cause the rocks to break down into smaller and smaller particles. These particles arc then taken along by water and deposited to far away places from their parent rock.
Wind Strong winds erode rocks down. They also carry sand from one place to the other like water does.
Biological weathering
Weathering of rocks by biological components like animals, plants and microbes is known as biological weathering. Organisms like lichens and mosses grow on the surface of rocks. While growing, they release certain substances that erode die rock surface to powder and form a thin layer of soiL When other small plants like moss, grow on this surface, they further break it down.
The roots of big trees go into cracks in the rocks and as the roots grow bigger, the cracks become wider, leading to weathering.
Chemical weathering
There are many chemical substances present in rocks, like sulphates, chlorides and phosphates of calcium, potassium and magnesium. During weathering, these chemicals are converted into solution and make the rocks porous, leading to further disintegration. Water also hydrolyses certain minerals in rocks, causing weathering.
Components of soil
Let us perform an activity to study various components of soil.
ACTIVITY-10
To observe the various components present in soil
You will need
Beaker, soil, water, spatula
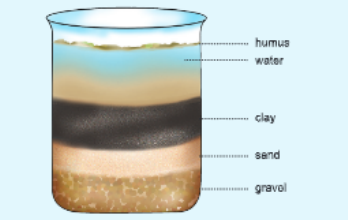
Procedure
Take some soil and put it in a beaker containing water. Ensure that the quantity of water is at least five rimes more than the quantity of soil.
Stir the soil and water in beaker vigorously tor some time with the help of a spatula and then allow the soil to settle down. Observe after some time.
In Activity 10, you have seen that soil is a mixture of various components. It contains following things
small particles of rock,
decayed living organisms called humus on the surface, and
various forms of microscopic life (microorganisms)
The average size of particles found in the soil decides what type of soil it is. Amount of humus and microscopic organisms found in it decides the quality of the soil.
Observations
What do you observe?
Inference
Observation
- After settling down did the soil at the bottom of the beaker become homogenous or layers were formed?
- If layers were formed, was each layer different from other? How?
- Was there anything floating on the surface of the water?
- Do you think some substances would haw dissolved in the water? How can you check dais?
inference
- Different layers v/oro formed.
- Each layer was different from the other. Each layer had different sized Soil particles.
- Yes, humus was floating on the surface.
- Some substances such as minerals would have dissolved in water.
- This can be checked by performing certain chemical tests.
Humus plays an important role in deciding the soil structure. This is because humus makes the soil more porous and allows water and air to penetrate deep underground.
The mineral nutrients that are found in a particular soil depend on the rocks from which it has been formed.
Different soils are suitable for growing different types of plants. Some of the factors that decide which plants will grow in which type of soil are
nutrient content of the soil,amount of humus present in it and depth of the soil.
Therefore, the topmost layer of the soil which contains humus and living organisms along with soil particles is called the topsoil. Hie quality of rhe topsoil is an important factor that decides biodiversity in an area.
Soil pollution
Removal of useful components from the soil and addition of other substances, which adversely affects the fertility of the soil and kills the diversity of organisms living in it, is called soil pollution.
Causes of soil pollution
- Soil pollution is mainly caused by the following
- Raw manure (farm and animal manure containing pathogens)
- Agricultural waste (chemical fertilizers and pesticides)
- Industrial waste (fly ash, metallic ash, etc.)
- Domestic waste (paper pulp, plastic, polythene bags, rubber, discarded gadgets, glass, metal scrap, etc.)
- The fertility of any type of soil is its capacity to sustain plant life with the nutrients it needs. However, modem farming practices use large amounts of fertilizers and pesticides.
- Continuous use of these substances over a long period of time can kill the soil microorganisms which recycle nutrients in the soil It also kills the earthworms, which are essential in making the humus. This destroys the soil structure and its fertility.
Natural Resources Chapter Summary Class 9
Prevention of soil pollution
- Soil pollution can be prevented by o judicious use of fertilizers and pesticides,
- controlling the release of effluents from industries into the soil and
- using safe methods of disposal of raw manure and domestic waste.
Soil erosion
Soil erosion also plays a role in the reduction of soil fertility. The removal and transportation of the top layer of soil from its original position to another place by flowing water or wind is called soil erosion.
Causes of soil erosion
The fine particles of soil may be carried away by flowing water or wind. This exposes the rocks underneath and leads to loss of a valuable resource because very little vegetation wall grow on the rock. Large-scale deforestation has also resulted in soil erosion. Topsoil that is devoid of vegetation is likely to be removed very quickly. This is found more commonly in hilly regions.
Thus, the causes of soil erosion may be strong winds, heavy rain, improper farming, dust storms, frequent floods and also indiscriminate human activities.
Once soil is eroded, it is very difficult to reverse the process of soil erosion.
ACTIVITY-11
To study the effect of flowing water on topsoil Yon will need
Two identical trays, soil, mustard or green gram or paddy seeds, water, beakers.
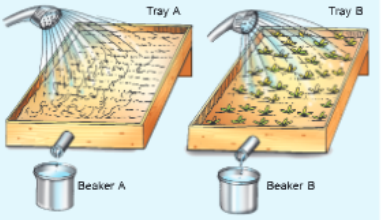
Procedure and Observations
Take two identical trays and fill them with soil.
Plant mustard or green grant or paddy in one of the trays. Leave the other tray as such. Water both die trays regularly for £-10 days till seedlings start growing in the first tray.
Now, fix both the trays in such a position that the)’ are tilted at the same angle.
Pour eipal amount of water gently on both the trays such that it flows out of the trays. You may place beakers beneath the trays for collecting water and soil.
The amount of soil that is carried out of the trays in the two beakers. Is the amount same in both the trays?
Now from a height, pour three to four times the amount of water that you poured earlier on both the trays.
Study the amount of soil that is earned out of the trays in this case. Does the amount of soil remain same in both the trays?
Is the amount of soil, that is washed our, more, less or equal to the amount that was washed out in earlier case?
Conclusion
The above activity shows that the amount of soil washed out was more in the tray that did not have any plant growth (TYay A). Similarly, the amount of soil washed out was more in the second case when more amount of water was poured from a height. This is because water frilling with a force erodes more soil particles and causes more soil erosion.
Prevention of soil erosion
From above activity, we may conclude that soil erosion can be checked by growing more trees on a barren land. This is because the roots of plants bind soil particles and prevent them from getting washed away with water or blown away by wind. On hillsides, if terrace farming (farming on slopes of Iiills by making small steps) is practised, soil erosion is slowed down. Along with checking soil erosion, vegetative cover on the ground also helps in the percolation of water into the deeper layers addition, sowing grasses, planting xerophytes, contour bunding (making soil elevation bunds) and making proper drainage canals around the fields also helps in preventing soil erosion.
BioGeoChemicalCycles-The Cycling of Material In The Biosphere
Energy alone is not sufficient to support life. Materials or chemical elements arc also necessary for the existence and survival of life. There are about 30-40 such elements required by living organisms for synthesizing their protoplasm, growth and development Life depends upon the availability of solar energy and also on the cycling of biogenic elements.
These biogenic nutrient elements flow from non living tilings to living ones and then back to non-living ones in a more or less circular path.
A continuous interaction between the biotic and abiotic components of the biosphere makes it a dynamic, but stable system. Thus, we can say that the circulation of matter or biogenic nutrient elements like carbon, hydrogen, oxygen, nitrogen, phosphorus, calcium, water and energy between biotic (living) world and abiotic (physical/non-living) world is biown as the biogeochemical cycle.
Characteristics of biogeochemical cycle
In biogcochcmical cycles, materials arc not lost but recycled.
There is regular circulation of biogenic nutrient elements between abiotic and biotic component of the biosphere.
It operates through non-living world (air, water, soil) and living world.
Decomposers help in the recycling of materials. They convert nutrients into usable forms.
It helps in maintaining nutrient pool of the earth. Let us study the processes involved in the following biogeochemical cycles Hydrological (water) cycle, Nitrogen cycle, Carbon cycle and Oxygen cycle.
Water Or Hydrological Cycle
Water cycle is the circulation of water within the earth’s hydrosphere which involves continuous exchauge of water between the atmosphere, land, surface and groundwater and living beings. In other words, the whole process of evaporation of water, its falling on land as rains, and later its (lowing into the sea via rivers is known as water cycle.
Steps of water cycle
- Evaporation It is the transfer of water from the surface of waterbodies like rivers, oceans, etc. into the atmosphere as water vapour. As a result of evaporation, water changes from the liquid to the gaseous phase. Water evaporates from oceans, ponds, lakes, ground, plants (during transpiration) and animals (as sweat and through respiration).
- Condensation The water vapour being lighter, rise up and condense to form tiny water droplets. These tiny droplets round up around the dust particles available in the atmosphere and form clouds. Precipitation The water vapour that condense to form clouds precipitate to form rain and snow.
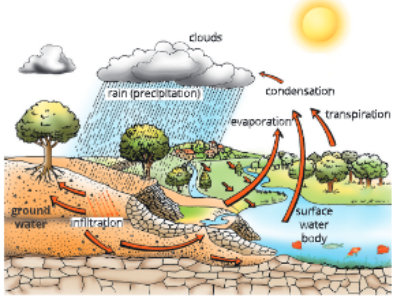
- When clouds are cooled due to rising up, the small droplets in them cool further and they come closer to each other. Many droplets combine to form big drops of water. These drops are so big that they can no longer float in air and fall down on the earth as rain. This rainwater falls in oceans as well as on land surface.
- Infiltration All the rainwater falling on the land does not flow to the sea. Some of it seeps into soil and becomes part of the underground reservoir of fresh water.
- Some of the groundwater finds its way to the surface through springs or we draw it out mechanically for our use through wells or tube wells. Water is also used by plants tor growth and photosynthesis, and animals for various life processes.
- Water is also capable of dissolving many substances. As water falls on rock surfaces or flow’s through rocks, it dissolves soluble minerals from it. This water then flows into river and ultimately reaches sea. Thus, many nutrients dissolved in water reach sea and are used by marine organisms such as fish.
Nitrogen Cycle
Earth’s atmosphere contains about 78% of nitrogen gas. Nitrogen is an essential component of many molecules necessary for life such as proteins, some vitamins and nucleic acids (DNA and RNA). It is also found in many other biologically important compounds such as alkaloids and urea.
In the atmosphere, it exists in the molecular form (N2) and some oxides. Thus, nitrogen is an essential component of life and life will become very simple if all the living beings could use the atmospheric nitrogen directly. However, living organisms with the exception of blue-green algae and few nitrogen¬fixing bacteria cannot use nitrogen directly or convert the comparatively inert nitrogen molecule into nitrates (NO3) and nitrites (NO2) which can be directly taken up and used to make the required molecules. It needs to be first converted into nitrates for use by the plants.
The cyclic process by which nitrogen is circulated continuously between the living and non-living components of the biosphere is called the nitrogen cycle.
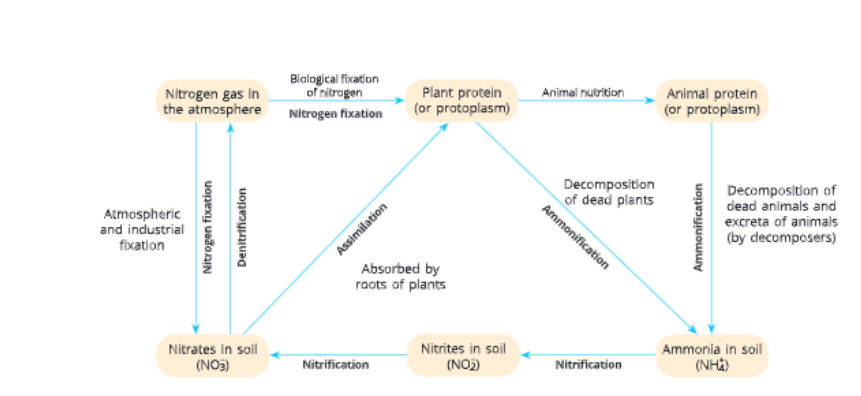
Steps of nitrogen cycle Nitrogen fixation
The process of converting free nitrogen of the atmosphere into nitrogen compounds is called nitrogen fixation. It takes place in two ways
Atmospheric nitrogen fixation Biological nitrogen fixation
Atmospheric nitrogen fixation
During lightning in the sky, when high temperature and pressure are created in the air, the nitrogen gas present in die atmosphere reacts with oxygen to produce oxides of nitrogen. These oxides of nitrogen dissolve in rainwater forming dilute nitric and nitrous acids, and fall on the land along with rainwater. These nitric and nitrous acids react with the alkalis of the soil (like limestone) to turn into nitrates, which are utilized by various plants.
N2+02->2N0 2NO + 02 —»2N02 4N02 + 2H20 + 02 4HN03
CaCOj + 2HN03 Ca(N03)2 + HzO + C02
Biological nitrogen fixation
It is the conversion of atmospheric nitrogen into nitrogen compounds by nitrogen-fixing bacteria.

Nitrogen-fixing bacteria can be free-living like Azotobacter and Clostridium, or symbiotic like Rhizobium. Uhese bacteria live in the root nodules of dicot leguminous plants and can fix atmospheric nitrogen into nirrates.
Certain blue-green algae like Anabaerta and Nostoc and non- leguminous plants like Ginkgo can also fix atmospheric nitrogen into nitrates.
KSEEB Class 9 Biology Important Questions Chapter 5
Nitrogen assimilation
The process of conversion of inorganic nitrogen compounds (ammonia salts and nitrates) into organic compounds (amino acids, nucleotides, etc.) that become a part of living organisms is called nitrogen assimilation. Plants absorb nitrogen compounds like nitrates and nitrites from the soil and water, and convert them into amino acids that in turn form plant proteins. Other complex organic compounds containing nitrogen are also formed by using some other biochemical pathways.
These proteins and complex compounds arc subsequently consumed by animals.
Ammonification
The process of conversion of complex organic compounds like proteins of dead and decaying organisms into ammonia is called ammoniiication. Once the animals and plants die, the dead remains of plants and animals are converted into ammonia, carbon dioxide and water by the action of putrefying bacteria, actinomycetes and fungi (decomposers) present in the soil and water.
Nitrification
The process of conversion of ammonia into nitrites and nitrates is called nitrification. Nitrification is brought about by some nitrifying bacteria present in the soil. Nitrates from the soil are absorbed by the plants. Energy is yielded in the process which is used by these bacteria.
Nilrosomotuts Ntlrobacter
bacteria. . „ , bacteria .
Ammonia Nitrites Nitrates
(NHJ) (NO 2) (NOS)
Denitrification
The conversion (degradation) of nitrate and nitrite salts to elemental nitrogen is called denitrification. It is carried out in the soil by free-living bacteria called Pseudomonas.
Thus, nitrogen passes through various steps, first from its elemental form in the atmosphere into simple molecules in soil and water, which in turn get converted to more complex molecules in living beings and then finally back again to simple nitrogen molecules in the atmosphere. This is known as nitrogen cycle.
Carbon Cycle
The cyclic process in which carbon is circulated continuously between the living and non-living components of the biosphere is called carbon cycle.
Carbon is found in many forms on the earth.
In its elemental form, it occurs as diamond and graphite.
In its combined form, it is found as carbon dioxide which is the main form in which it is present in the atmosphere.
Carbon is also found as carbonate and hydrogen carbonate salts in various minerals.
Carbon is a basic constituent of all life forms, hr fact, carbon is the most essential constituent of all the major organic compounds like carbohydrates, proteins, fats, vitamins, enzymes and nucleic acids present in living organisms.
Tire endoskeleton and exoskeleton of various animals are also formed from carbonate salts.
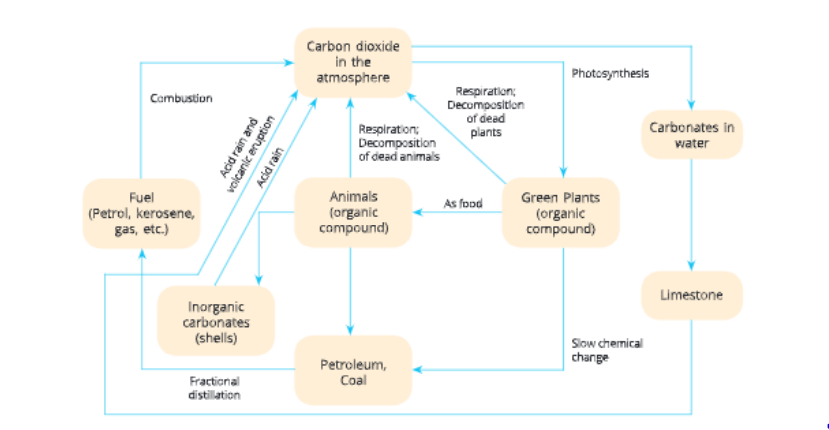
Steps of carbon cycle
Through photosynthesis In life forms, carbon is incorporated through photosynthesis. Carbon is present as carbon dioxide gas in the atmosphere. Green plants use this carbon dioxide and prepare their food by the process of photosynthesis. Photosynthesis takes place in the presence of sunlight by green plants that contain chlorophyll. During this process, carbon dioxide changes into glucose (carbohydrate) molecules. These glucose molecules are then either converted into other carbohydrates like starch or are used to provide energy during respiration or oxidation for the synthesis of other biologically important molecules. When animals cat the plant, plant carbohydrate is
converted into animal carbohydrate.
Carbon cycle also involves respiration. When plants and animals respire, they convert glucose into energy and in turn give out carbon dioxide, which is returned to die atmosphere. When animals and plants die, their bodies are decomposed by decomposers and carbon dioxide is returned to the atmosphere.
By combustion Process of combustion also adds carbon dioxide to the atmosphere. Some of die dead plants and animals get buried deep under the earth. Under high pressure and temperature, these change into fossil fuels like coal and petroleum through slow chemical changes over millions of years. Petroleum gives us fuels like kerosene, petrol, diesel, petroleum gas, etc. When these fuels are burnt to provide energy for various needs like heating, cooking, transportation and industrial processes, they give out carbon dioxide which goes into the atmosphere. In feet, the percentage of carbon dioxide has doubled since die industrial revolution due to the burning of fossil fuel on a mammoth scale.
Some carbon dioxide is present in dissolved state in water. This gets converted into calcium carbonate (CaC03) in limestone and other carbonate rocks. Weathering of carbonate containing rocks and treatment of their minerals gives carbon dioxide. When add rain falls on diese rocks, dien carbon dioxide is released.
Volcanic eruptions and hot springs also release carbon dioxide into the atmosphere.
Thus, there is a continuous exchange of carbon dioxide between atmosphere, waterbodies and living beings through physical and biological activities.
Green House Effect And Global Warming
You observed in Activity 1 diat heat was trapped by glass and hence die temperature inside a glass enclosure was much higher than the surroundings. This phenomenon in which temperature inside die glass chamber was higher than outside was used to create an enclosure having higher temperature inside where tropical plants could be kept warm during winters in colder climates. Such enclosures made of glass or thick plastic sheets for growing delicate plants are called greenhouses.
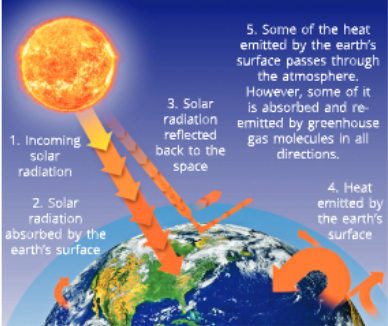
We know’ that sun rays pass through the earth’s atmosphere and some of diem are reflected back into space. Thus, most of the sun rays that are absorbed warm die earth’s surface. There arc some gases such as carbon dioxide which prevent the escape of heat from the eardi. An increase in die percentage of such gases in the atmosphere would increase the average temperature worldwide. This is called the greenhouse effect. Methane and carbon dioxide are considered as the greenhouse gases. An increase in the carbon dioxide or methane content in die atmosphere causes more heat to be trapped and retained by the atmosphere, leading to greenhouse effect.
The increase in temperature of the earth’s surface and lower atmosphere due to greenhouse effect is called global wanning. It would cause melting of continental and mountain glaciers and thus, would cause flooding of coastal areas of some countries. It would also bring about climate change, thereby increasing the chances of cyclones, hurricane and floods. There would be higher incidence of diseases as well due to global warming.
KSEEB SSLC Chapter 5 Notes Detailed Explanation
Oxygen Cycle
Like carbon and nitrogen, oxygen is also a basic clement of life. It is a very abundant element on our earth. In elemental form in atmosphere, it is found to the extent of 21 per cent In addition to constituting about 21 per cent of the atmosphere, oxygen also occurs in combination as oxides in the earth’s crust, in carbon dioxide and in water. It occurs as oxides of most metals and silicon in the earth’s crust and also as carbonates, sulphates, nitrates and other minerals. Mosr biological molecules like carbohydrates, flits, nucleic acids and proteins contain oxygen.
Oxygen enters the living or biotic world through the process of respiration, in which energy is released from die food material. Beside the most obvious uses in breathing and respiration, oxygen is vital for us in man)’ ways. Dissolved oxygen in water supports aquatic life. It is also needed for decomposition of organic waste by aerobic bacteria. In addition, in the form of ozone, it provides protection to life from the UV rays of sun.
Although oxygen has so many uses and is necessary for life in the process of respiration, you may be interested to learn that some bacteria are poisoned by elemental oxygen. In fact, even the process of nitrogen fixation by bacteria, which you have learnt in previous section does not take place in the presence of oxygen. But have you ever wondered from where do we 130 get oxygen? How does it keep on renewing itself?
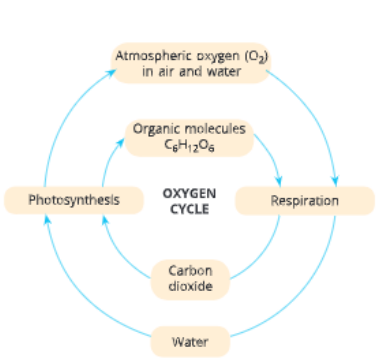
The cycle that maintains the levels of oxygen in the atmosphere is known as oxygen cycle.
Oxygen is used up in the atmosphere by three processes, namely
- combustion,
- respiration and
- formation of oxides of nitrogen
However, oxygen is returned to the atmosphere by autotrophs during photosynthesis. The concentration of oxygen in the air and water is maintained since the rate of its release during photosynthesis and use during respiration remain almost the same.
OzoneLayer Deplection
What is ozone layer?
You have read that elemental oxygen is found in the form of a diatomic molecule. However, in the upper part of die atmosphere, oxygen is present in the form of ozone. Ozone is an allotrope of oxygen. It is made of 3 atoms of oxygen (triatomic) in comparison to diatomic oxygen (02). Ozone is poisonous. However, it is not stable near the earths surface. Ozone is present mostly in the stratosphere and its maximum concentration occurs at a height of23-25 km above the equator or at slightly lower height at other places.
What are the ill effects of ozone layer depletion?
At ground level, ozone is a harmful pollutant that damages plants and building materials, and is hazardous to human health. However, in die upper atmosphere, ozone is very important and acts like a life cover. It protects us by absorbing the dangerous ultraviolet (UV) rays coming from the sun. Without the ozone layer, organisms on the earth would be subjected to life threatening radiations from sun.
Ozone Layer Depletion – Ozone Hole
- Recently, the British Antarctic Atmosphere Survey (BAAS) announced a startling and disturbing discovery – the ozone layer was depleting over the South Pole of the earth. In 1993, about 70% of the Antarctic region ozone was destroyed, which is similar to over an area about the size of North America.
- The ‘ozone hole’ is increasing every year. It is difficult to imagine the consequences for life on tire earth if ozone layer depletion continues further.
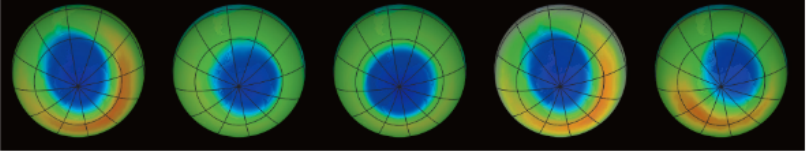
What causes ozone layer depletion?
- There arc many man-made compounds such as CFCs which are found persisting in the atmosphere. These are carbon compounds having both fluorine and chlorine, which are very stable and cannot be degraded by any biological processes.
- Since, CFCs are very stable molecules, they persist for decades, even centuries, once released. When they diffuse into the atmosphere, they react with the UV radiations from the sun and release chlorine atoms that destroy ozone. This results in the reduction of the ozone layer.
Summary
- Natural resources like air, water, soil, etc. are useful to mankind as they provide us with food, clothes and shelter.
- The life supporting zone of the earth is called biosphere. There are mainly three zones of biosphere, namely lithosphere, hydrosphere and atmosphere.
- Air is used for respiration, combustion, moderating temperature and bringing rains.
- An undesirable change in the physical, chemical and biological characteristics of our surroundings, which has harmed human life and other living beings is known as pollution.
- Air pollution may be defined as the occurrence of foreign particles, gases and other materials in air, which have adverse effects on biological communities and physical surroundings.
- Carbon monoxide, carbon dioxide, oxides of nitrogen and sulphur, smog, suspended particulate matter and pesticides are some common air pollutants.
- Uneven heating of air over land and waterbodies causes wind.
- Rains are formed due to evaporation of water from waterbodies and subsequent condensation.
- All living beings need water to carry out various life processes. Water is the prime constituent of all living cells.
- Water may be polluted by pesticides, chemicals, and industrial and domestic waste.
- Soll is formed mainly by weathering of rocks by wind, water and rise and fall in temperatures. Blological components also help in soil formation.
- Circulation of matter or nutrients and energy between biotic and abiotic components is known as biogeochemical cycle.
- The process of converting free nitrogen of the atmosphere into nitrogen compounds is called nitrogen fixation.
- Azotobacter, Clostridium and Rhizobium are nitrogen fixing bacteria.
- In the upper atmosphere, ozone acts like a life cover that protects us by absorbing the dangerous ultraviolet rays of the sun. Man-made compounds such as chlorofluorocarbons have caused a hole in the ozone layer thus depleting it.
