KSEEB Solutions For Class 9 Science Chapter 3 Atoms and Molecules Important Concepts
Atoms and molecules, law of constant proportions, atomic and molecular masses, mole concept, valency and chemical formula of common compounds.
Atom
It is the smallest particle which takes part in chemical reaction. Example: Na, K, Fe, etc.
Molecule
It is made up of two or more atoms and it is capable to exist independently. Example: H2,Cl2,O2 etc
Atomicity
The number of atoms present in one molecule of a substance is called its atomicity.
Examples: Monoatomic, diatomic, triatomic, tetra atomic.
Read and Learn More KSEEB Solutions for Class 9 Science
Ion
An atom or group of atoms which carries positive or negative charge is called an ion.
Ionic compounds
The compounds consisting of cations and anions are called ionic compounds.
Cation
It is a positively charged ion.
Example: Na+, K+, Ca2+, Mg2+ etc
KSEEB Solutions for Class 9 Science Chapter 3
Anion
It is a negatively charged ion.
Example: Cl , Br~, F~, O2” etc
| Class 9 Social Science | Class 9 Science | Class 9 Maths |
Monoatomic
They consist of only one atom.
Example: H, He, S, etc
Diatomic
They consist of two atoms. Example: H2,02, N2, etc.
Triatomic
They consist of three atoms. Example: O3
Tetra atomic
They consist of four atoms. Example: P4
Law of conservation of mass
It states that matter can neither be created nor be destroyed in a chemical reaction.
Dalton’s atomic theory
All matter whether element, compound or mixture is composed of small particles called atoms.
Atomic mass unit
It is defined as the mass of 1/12th of the mass of 1 atom of car bon-12 isotope.
KSEEB Class 9 Science Atoms and Molecules Solutions
Mole concept
Mole is counting unit for atoms, molecules or ions and is equal to 6.022 x1023.
This number is called Avogadro’s constant and is denoted by symbol N.
Mole
The amount of a substance which contains as many particles (atoms, ions or molecules) as in 12 g of C-12. 1 mole = 6.022 x -1023 atoms.
Gram atomic mass
Atomic mass of a substance expressed in grams is called its gram atomic mass. This amount is called one gram atom.
Gram molecular mass
Molecular mass of a substance expressed in grams is called gram molecular mass. This amount is called one gram molecule.
Valency of an element
The combining capacity of the element.
Atoms And Molecules Exercises
Question 1. A 0.24g sample of compound of Oxygen and Boron was found by analysis to contain 0.096g of Boron and 0.144g of Oxygen. Calculate the percentage
composition of the compound by weight.
Answer Percentage of any element in a compound Mass of the element
\(=\frac{\text { Mass of the element }}{\text { Mass of the compound }} \times 100\)
Percentage of Oxygen
\(=\frac{\text { Mass of Oxygen }}{\text { Mass of compound }} \times 100\)
\(=\frac{0.144}{0.24} \times 100=60 \%\)
Percentage of boron
\(=\frac{\text { Mass of Boron }}{\text { Mass of compound }} \times 100\)
Question 2. When 3.0g of Carbon is burnt in 8.0g of Oxygen, ll.OOg of Carbon dioxide is produced. What mass of Carbon dioxide will be formed when 3.00g of Carbon is burnt in 50.00g of Oxygen? Which law of chemical
combination will govern you answer?
Answer 3g of Carbon produce Carbon dioxide=l 1 g The remaining oxygen 50g – 8g = 42g does not take part in the reaction. The law of definite proportion is governed by the above data.
Question 3. What are polyatomic ions? Give examples.
Answer Polyatomic ions: Two or more different atoms unite to form a charged particle and are called polyatomic ions.
Example: (PO4)-3 Phosphate, Nitrate(NG3)—1
Question 4. Write the chemical formulae of the following,
1. Magnesium chloride
Answer
\(\mathrm{Mg}^{2+}+\mathrm{Cl}^{-1} \rightarrow \mathrm{Mg}^{2+} \mathrm{Cl}^{-1}\)
2. Calcium oxide
Answer
\(\mathrm{Ca}^2+\mathrm{O}^2 \rightarrow \mathrm{Ca}^{2+} \mathrm{O}^{2-}\)
3. Copper nitrate
Answer
\(\mathrm{Cu}^{2+}+\mathrm{NO}_3^{-1} \rightarrow \mathrm{Cu}^{2+}+\mathrm{NO}_3^{-1}\)
4. Aluminum chloride
Answer
\(\mathrm{Al}^{3+}+3 \mathrm{Cl} \rightarrow \mathrm{Al}^{3+} \mathrm{Cl}^{-1}\)
5. Calcium carbonate
Answer
\(\mathrm{Ca}^{2+}+\mathrm{CO}_3^{2-} \rightarrow \mathrm{Ca}^{2+} \mathrm{CO}_3^{2-}\)
Question 5. Give the names of the elements present in the following compounds:
1. Quicklime
Answer CaO – Elements present are calcium and oxygen
2. Hydrogen Bromide
Answer HBr – Elements present are hydrogen and Bromide
3. Baking powder
Answer NaHC03 – Elements present are Sodium hydrogen carbon and oxygen
4. Potassium Sulphate
Answer
\(\mathrm{K}_2 \mathrm{SO}_4-\)Elements present are potassium, sulphur and oxygen

Karnataka Board 9th Science Chapter 3 Notes PDF
Question 6. Calculate the molar mass of the following substances.
1. Ethyne \(\mathrm{C}_2 \mathrm{H}_2\)
2. Sulphur molecule S8
3. Phosphorus molecule P4(Atomic Mass of P=31) Hydrochloric acid, HCl
Nitric acid \(\mathrm{HNO}_3\)
Answer:
1. Molecular mass of \(\mathrm{C}_2 \mathrm{H}_2\) (Ethyne)
= 2 x atomic mass of C + 2 x atomic mass of H
= 2 x 12 + 2 x 1 =26g
2. Molecular mass of S (Sulphur)
= 8 x atomic mass of S
= 8 x 32 = 256g
3. Molecular mass of P4 (Phosphorus)
= 4 x atoinic mass of P
= 1×31 = 124g
4. Molecular mass of HCl (Hydrochloric acid) = 1 x atomic mass of H + 1 x atomic mass of Cl
= 1 x 1 +35,5 = 36.5g
5. Molecular mass of HN03 (Nitric acid)
= 1 x atomic mass of H + 1 x atomic mass of
N + 3 x atomic mass of O
= 1 X 1 + 1 X 14 + 3 x 16 = 63g
Question 7. What is the mass of
1. 1 mole of Nitrogen atom
2. 4 moles of Aluminium atom (Atomic mass of A1 = 27)
3. 10 moles of Sodium Sulphite \(\left(\mathrm{Na}_2 \mathbf{S O}_3\right)\)
Answer:
1. 1 mole of nitrogen atom
= 1 x gram atomic mass of nitrogen atom
= 1 x 14= 14g
2. 4 moles of Aluminium atoms
= 4 x gram atomic mass of Aluminium atoms
= 4x 27= 108g
3. 10 moles of Sodium Sulphite \(\left(\mathrm{Na}_2 \mathbf{S O}_3\right)\)
= 10 (2 x gram atomic mass of Na + 1 x gram 1
atomic mass of Sulphur + 3 x gram atomic mass of oxygen)
= 10 (2 x 23 + 1 x 32 + 3 x I6)g
= 10 (46g + 32g + 48g)
= 10x 126g=1260g
Question 8. Convert into mole
1. 12g of Oxygen gas
2. 20g of Water
3. 22g of Carbon dioxide
Answer
1. Number of moles \((\mathrm{n})=\frac{\text { Given Mass }(\mathrm{m})}{\text { Molar Mass }(\mathrm{M})}\)
Molecular mass of O2 = 2’x 16 = 32g
32g o f Oxygen = 1 mole
\(12 \mathrm{~g} \text { of Oxygen }=\frac{12}{32} \text { mole }\)
= 0.375 mole
2. Molar mass of \(\mathrm{H}_2 \mathrm{O}\) = 2 x Igx 1 x 16g= 18g
18g of Water = 1 mole
\(20 \mathrm{~g} \text { of Water }=\frac{20}{18} \text { mole }\)
= 1,11 mole
3. Molar mass of \(\mathrm{CO}_2\)= 1 x 12g + 2 x 16g = 44g
44g of Carbon dioxide = 1 mole
\(22 \mathrm{~g} \text { of Carbon dioxide }=\frac{22}{44} \text { mole }\)
= 0.5 mole
Question 9. What is the mass of
1.0.2 mole of Oxygen atoms
2. 0.5 mole of Water Molecules
Answer
1. 1 mole of Oxygen atoms
= 1 x 16= 16g
0.2 mole of Oxygen atoms
= 16g x .0.2 = 3.2g
2. 1 mole of Water (H20) molecules
=2 x lg+ 1 x igg = 18g
0.5 mole of Water \(\left(\mathrm{H}_2 \mathrm{O}\right)\)molecules
= 18g x 0.5 = 9.0g
Question 10. Calculate the number of molecules of Sulphur
\(\left(S_8\right)\)present in 16g of solid sulphur.
Answer
\(\text { Number of moles }=\frac{\text { Given Mass }(\mathrm{m})}{\text { Molar Mass }(\mathrm{M})}\)
1 mole of Sulphur (Sx)
= 8><1 gram atomic mass of Sulphur
= 8 x 32 g = 256g
1 mole of S„ molecules
= 6.022 x 1023 molecules
256g of molecules has
= 6.022 x \(10^{23}\)molecules
16g of molecules has
\(=\frac{6.022 \times 10^{23} \times 16}{256} \text { molecules }\)
= 3.76x 1022moiecules.
Class 9 Science Chapter 3 KSEEB Solutions PDF
Question 11. Calculate the number of Aluminium ions
present in 0.051g of Aluminium Oxide \(\left(\mathrm{Al}_2 \mathrm{O}_3\right)\) (Hint: The mass of an iron is the same as that of an atom of the same element. Atomic mass of AI = 27 u.)
Answer
1 gram molecule (1 mole) of \(\mathrm{Al}_2 \mathrm{O}_3\)
= 2 x gram atomic mass of Al + 3 x gram
atomic mass of O
= 2 x 27g + 3 x I6g
= 54g+48g= 102g
1 gram molecule (1 mole) of A1203 contains Aluminum ions
= 2 x 6.022 x 1023
= 12.044 x 1023
102g of Al2 O 3 has number of Aluminium ions
= 12.044 x 1023
0.05 lg of has number ofAluminium ions
\(=\frac{12.044 \times 10^{23} \times 0.051}{102}\)
Atoms And Molecules Textual Questions
Question 1. In a reaction, 5.3g of Sodium Carbonate reacted with 6g of Ethanoic acid. The products where 2.2g of Carbon dioxide, 0.9g of water and 8.2g of Sodium Ethanoate. Show that these observations are in agreement with the law of conservation of mass. Sodium Carbonate + Ethanoic acid sodium > Ethanoate + Carbon dioxide + Water
Answer
Total mass of the reaction equals of Sodium Carbonate + Mass of Ethanoic acid solution.
= 5.3g + 6g= 11.3g
Total mass of products = Mass of sodium ethanoate solution + Mass of carbon dioxide
= 8.2g + 2.2g+0.9g=U.3g
The mass of reactants is equal to the mass of products, therefore, it proves law of conservation of mass.
Question 2. Hydrogen and oxygen combine in the ratio 1: 8 by mass to form water. What mass of Oxygen gas would be required to react completely with 3g of hydrogen gas?
Answer As hydrogen and oxygen combine in the ratio 1: 8 by mass, this means that lg of hydrogen combines with 8g of oxygen.
3g of hydrogen will react with oxygen
= 8 x 3g = 24g
Question 3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer The following postulate of Dalton’s atomic theory is a result of the law of conservation of mass. “Atoms are indivisible particles which cannot be created or destroyed in a chemical reaction.”
Question 4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer “The relative number and kinds of atoms are constant in a given compound.”
Question 5. Define the atomic mass unit.
Answer Themassof l/12ftpartof C-12 is equivalent to one atomic mass unit.
Question 6. Why is it not possible to see an atom with. naked eyes?
Answer Because an atom is too small the atomic radii of an atom is of the order 10’10mtol0’9m
Question 7. Write down the formulae of the following.
1. Sodium oxide
2. Aluminium chloride
3. Sodium sulphide
4. Magnesium hydroxide
Answer
1. Sodium oxide —>\(\mathrm{Na}_2 \mathrm{O}\)
2. Aluminum chloride —>\( \mathrm{AlCl}_3\)
3. Sodium sulphide—>\(\mathrm{Na}_2 \mathrm{~S}\)
4. Magnesium hydroxide —>\(\mathrm{Mg}(\mathrm{OH})_2\)
Question 8. Write the names of the compounds represented by the following formulae.
1\(\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3\)
2.\(\mathrm{CaCl}_2\)
3.\(\mathrm{K}_2 \mathrm{SO}_4\)
4.\(\mathrm{KNO}_3\)
5.\(\mathrm{CaCO}_3\)
Answer
- \(\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3\)—>Aluminium Sulphate
- \(\mathrm{CaCl}_2\)—> Calcium Chloride
- \(\mathrm{K}_2 \mathrm{SO}_4\)—>Potassium Sulphate
- \(\mathrm{KNO}_3\)—> Potassium Nitrate
- \(\mathrm{CaCO}_3\) —> Calcium Carbonate
Question 9. What is meant by the term chemical formula?
Answer The chemical formula of a compound is a symbolic representation of its composition and actual number of atoms in one molecule of a pure substance, maybe an atom or a compound.
KSEEB 9th Science Chapter 3 Important Questions
Question 10. How many atoms are present in a
1. \(\mathrm{H}_2 \mathrm{~S}\)molecule
2. \(\mathrm{PO}_4^{3-}\)ion
Answer
1. \(\mathrm{H}_2 \mathrm{~S}\)3 atoms are present.
2. \(\mathrm{PO}_4^{3-}\)5 atoms are present,
Mole Concept KSEEB Class 9 Textbook Solutions
Question 11. Calculate the molecular mass of Molecular mass of\( \mathrm{H}_2, \mathrm{O}_2, \mathrm{Cl}_2\) \(\mathrm{CO}_2, \mathrm{CH}_4, \mathrm{C}_2 \mathrm{H}_6, \mathrm{C}_2 \mathrm{H}_4, \mathrm{NH}_3, \mathrm{CH}_3 \mathrm{OH}\)
Answer
Molecular mass of
\(\mathrm{H}_2=2 \times\)atomic mass of H
= 2 x lg = 2g
\(\mathrm{O}_2\)= 2 x 16g = 32g
\(\mathrm{Cl}_2\)= 2 x 35.5 = 71 g
\(\mathrm{CO}_2\)-1 x 12g + 2 x 16g = 44g
\(\mathrm{CH}_4\)– 1 x 12g + 4 x 1 g = 16g
\(\mathrm{C}_2 \mathrm{H}_6\) = 2X 12g + 6x lg = 30g
\(\mathrm{C}_2 \mathrm{H}_4\) = 2X 12g + 4x lg = 28g
\(\mathrm{NH}_3\)= 1 x 14g + 3 x lg= 17g
\(\mathrm{CH}_3 \mathrm{OH}\)= l X 12g + 4 X lg+ lx 16g = 32g
Question 12. Calculate the formula unit masses of ZnO, Na20, K2C03. Given atomic masses of (Zn 65u, Na = 23u, K = 39u, C = 12u and 0 = 16 u)
Answer The formula unit mass of ZnO
= 1 x atomic mass of Zn x 1 x atomic mass of C
= 1 x 65 + 1 x 16 = 81u
The formula unit mass of\(\mathrm{Na}_2 \mathrm{O}\)
= 2x 23 + 1x 16 = 62u
The formula unit mass of\(\mathrm{K}_2 \mathrm{CO}_3\)
= 2x 39+1 x 12 + 3x 16 = 138u
Question 13. If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?
Answer
l mole (6.022 x10^{23} atoms) of Carbon weighs
= 12g
1 atom of 1 carbon weighs =\(\frac{12}{6.022 \times 10^{23}}\)
=1.99 x 10’23g
Question 14. Which has more number of atoms, 100 grams of Sodium or 100 grams of Iron?
(Given atomic mass of Na = 23u, Fe = 56u)
Answer 23g of sodium has atoms = N
100g of Sodium has atoms =\( \frac{100}{23} \mathrm{~N}_0\)
= 4.3 \(\mathrm{N}_0\)
56g of Iron has atoms = N
100 g of Iron has atoms =\(\frac{100}{56} \mathrm{~N}_0\)
= 1.78 N.
1oog of Sodium has more atoms than l00g of Iron.
Atoms And Molecules Additional Questions
Question 1. Classify each of the following on the basis of their atomicity.
1. \(\mathrm{NO}_2\)
2. \(\mathrm{He}\)
3. \(\mathrm{H}_2 \mathrm{O}_2\)
4. \(\mathrm{CH}_4\)
Answer:
1. Triatomic
2. Monoatomic
3. Tetra atomic
4. Renta atomic
Question 2. What do you understand by 1 amu or lu?
Answer 1 amu or lu stands for 1/12th of the mass of an atom of Carbon-12 isotope.
Question 3. What is the difference between an atom and a molecule?
Answer Atom is the smallest particle of an element that may or may not be capable of free existence. Molecule is the smallest particle of an element or compound which is capable of free existence.
Question 4. What do you understand by the ‘atomicity’ of a substance?
Answer: The number of atoms present in 1 molecule of a substance is called its atomicity.
KSEEB Class 9 Science Textbook Solutions Chapter 3
Question 5. A glass jar contains 1.7g of Ammonia gas. Calculate the following:
1. Molar mass of Ammonia
2. How many moles of ammonia are present in the glass jar?
Answer:
1. Molar mass of Ammonia = 14 + 3 = 17g
2. No of moles \(=\frac{\text { Given Mass }(\mathrm{m})}{\text { Molar Mass }(\mathrm{M})}=\frac{1.7}{17}\)
\(=\frac{1}{10}=0.1\)mole
Atoms And Molecules High-order Thinking Questions
Question 1. Which of the following species is electrically neutral and why? \(\mathbf{K}^{+}, \mathbf{C l}^{-}, \mathbf{S}^{2-}, \mathbf{K}\)
Answer K, because it has equal number of protons and neutrons.
Question 2. Give two examples to show that law of conservation of mass applies to physical change also.
Answer Law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. This law applies to physical changes.
Example: Melting of wax, melting of ice into water.
Question 3. Calculate the molecular mass of the following compounds.
\({ a. } \mathrm{H}_2 \mathrm{SO}_4\)
\({ b. } \mathrm{Na}_2 \mathrm{CO}_3\)
Answer
1. \(\mathrm{H}_2 \mathrm{SO}_4\)=2 x H + 1 x s I 4 x O
= 2 x 1 + 1 x 32 + 4 x 16-98u
\(\mathrm{Na}_2 \mathrm{CO}_3=\)2xNa+lxC + 3xO
= 2X23+1X]2 + 3X 16 = 106u
Question 4. Sample of Vitamin C is known to contain 2.58 x 1024 oxygen atoms. How many moles of oxygen atoms are present in the sample?
Answer: 1 mole of Oxygen atom = 6.023 x \(10^{23}\) atoms
Number of moles of oxygen atoms
\(=\frac{2.58 \times 10^{24}}{6.022 \times 10^{23}}=4.28\) mole
=4.28 moles of oxygen atoms.
Atoms And Molecules Unit Test Multiple Choice Questions
Question 1. 3.42g of sucrose is dissolved in 18g of water in a beaker. The number of oxygen atoms in the solution ______
1. 6.68 x\(10^{23}\)
2. 6.09x \(0^{22}\)
3. 6.022 x\(0^{23}\)
4. 6.022\(\times 10^{21}\)
Answer (1)
KSEEB Class 9 Science Chapter 3 Exercise Solutions
Question 2. The balancing of chemical equation is based on______
- Law of conservation of mass
- Law of combining value
- Law of constant proportion
- Avogadro’s law
Answer (1)
Question 3. Which of the following contains maximum number of molecules?______
- lg\(\mathrm{CO}_2\)
- lg\(\mathrm{N}_2\)
- lg\(\mathrm{H}_2[/atex]
- Ig[latex]\mathrm{CH}_4l[/atex]
Answer (3)
Atoms And Molecules True Or False
1. Sodium shows valency of [latex]\mathrm{Na}^2\) and\(\mathrm{Na}^{2+}\) False
2. Isotopes have same atomic mass. False
3. CO is carbon monoxide while CO is cobalt. True
Atoms And Molecules Answer The Following
Question 1. What is lu equal to?
Answer of mass of 1 atom ofC-12.
Question 2. Name the element used in filament of electric bulb.
Answer Tungsten
Question 3. An element X shows variable valencies of 3 and 5. What will be the formulae of its oxides?
Answer
\({ }_3^{\mathrm{X}}=\mathrm{X}_2 \mathrm{O}_3\)
\(\bar{X}_5^{\mathrm{X}}=\mathrm{X}_2 \mathrm{O}_5\)
Atoms and Molecules Class 9 KSEEB Question Answer
Question 4. Calculate the molecular mass of the following substances:
1. Ethyne \mathrm{C}_2 \(\mathrm{H}_2\)
2. Sulphur \(S_8\)
Answer
1. Ethyne \mathrm{C}_2 \(\mathrm{H}_2\)
Molecular mass of \(\mathrm{C}_2 \mathrm{H}_2\)= 2 x atomic mass of
C + 2 x atomic mass of H
= 2 xl2u + 2 x lu = 26u
2. Sulphur\(\mathrm{S}_8\)
Molecular mass of = 8 x atomic mass of Sulphur
= 8 x 32u =256u
Question 5. What is the difference between 2H and H2
Answer 2H represents two atoms of hydrogen and represents a molecule of hydrogen
Atoms And Molecules Answer The Following
Question 1. State the rules for writing the chemical formulae.
Answer
- The valency or changes on the iron must be balanced
- When a compound consists of a metal and a non-metal, the name or symbol of the metal is written first.
- In compounds formed with polyatomic ions, the iron is enclosed in a bracket before writing the number to indicate the ratio.
Question 2. Nagraj took 5 moles of carbon atoms in a container and Ramesh also took 5 moles of sodium atoms in another container of same weight
1. Who’s container is heavier?
2. Whose container has more number of atoms?
Answer
1. Mass of sodium atoms carried by Nagraj = (5 x23)g=115g
While mass of carbon atoms carried by Ramesh = (5 x 12)g = 60g
Thus, Nagraj container is heavy.
2. Both the containers have same number of atoms as they have same number of moles of atoms.
Numerical Problems On Mole Concept Class 9 KSEEB
Question 3. Write the cations and anions present in the following compounds.
Answer
Compound
1. \(\mathrm{CH}_3 \mathrm{COONa}\)
2. NaCl
3. \(\mathrm{NH}_4 \mathrm{NO}_3\)
Cation
1.\(\mathrm{Na}^{+}\)
2. \(\mathrm{Na}^{+}\)
3.\(\mathrm{NH}_4^{+}\)
Anion
1.\(\mathrm{CH}_3 \mathrm{COO}^{-}\)
2.\(\mathrm{Cl}^{-}\)
3.\(\mathrm{NO}_3^{-}\)
Karnataka Board 9th Science Chapter 3 MCQs
Question 4. State the atomicity of the following molecules,
Answer:
- Oxygen —» Diatomic
- Phosphorus —» Tetra atomic
- Sulphur —» Polyatomic
- Argon —> Monatomic
Atoms and molecules Activity
Question 1. The Law of Conservation of Mass.
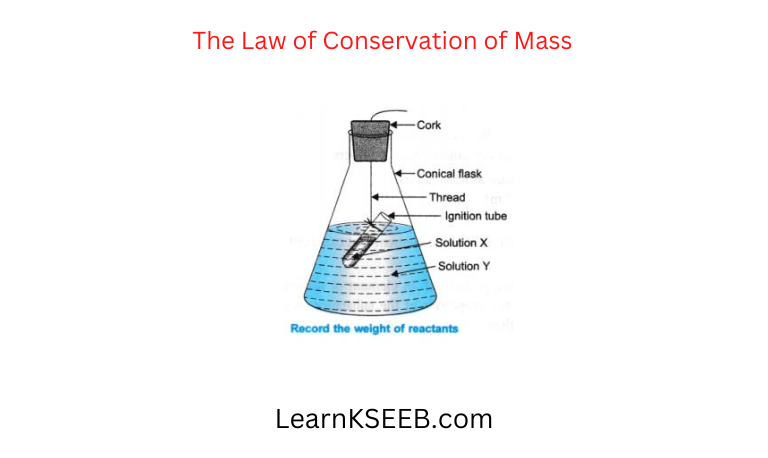
Atoms And Molecules Experimental Procedure
Prepare 5% solution of sodium sulphate and Barium Chloride. A little amount of sodium sulphate solution is taken in a conical flask and a little solution of Barium Chloride is taken in an ignition tube. Hang the ignition tube in the conical flask carefully as shown in the figure. Put a cork on the flask and weigh the flask with its contents. Now tilt and swirl the flask so that the two solutions mix up well. Weigh the flask again.
Observation: Chemical reaction takes place. Weight of the flask remains same before and after the reaction.
Conclusion: Total mass o f the products = Total mass of the reactants. Thus, the law of conservation of mass is proved.
