Units and Measurements Solutions KSEEB Class 11 Physics Chapter 2 Very Short Answer Questions
Question 1. Distinguish between accuracy and precision.
Answer:
Accuracy:
- It is defined as the closeness of the measured value to the true value.
- It depends on the minimization of errors.
Precision:
- It is defined as to what resolution the quantity is measured.
- It depends on the least count of the measuring instrument.
Read and Learn More KSEEB Class 11 Physics Solutions
Question 2. What are the different types of errors that can occur in a measurement?
Answer:
Types Of Errors
- Systematic errors and
- Random errors.
Systematic Errors Are Again Divided Into
- Imperfection errors
- Environmental errors and
- Personal errors.
KSEEB Class 11 Physics Chapter 2 Solutions PDF
Question 3. How can systematic errors be minimized or eliminated?
Answer:
Systematic Errors Can Be Minimised
- By improving experimental techniques,
- By selecting better instruments.

Question 4. Illustrate how the result of a measurement is to be reported indicating the error involved.
Answer:
The result of the measurement is in the form of a number which indicates the precision of measurement along with the unit of the same physical quantity.
Question 5. What are significant figures and what do they represent when reporting the result
of a measurement?
Answer:
Significant figures represent all practically measured digits plus one uncertain digit at the end.
When a result Is reported in this way we can know up to what extent the value Is reliable and also the amount of uncertainty In that reported value.
Question 6. Distinguish between fundamental units| and derived units.
Answer:
The units of fundamental physical| quantities are called fundamental units.
Example: Kg, m, sec, etc.,
The units of derived physical quantities are called derived units.
Example: m/sec, J, m/sec² etc.,
Question 7. Why do we have different units for the same physical quantity?
Answer:
To measure the same physical quantity we have different units by keeping the magnitude of the quantity to be measured.
Example:
- The measure astronomical distances we will use light year.
- 1 light year = 9.468 x 1015 m.
- To measure atomic distances we will use Angstrom Å (or) Fermi.
Question 8. What is dimensional analysis?
Answer:
Dimensional analysis is a tool to check the relations among physical quantities by using their dimensions.
Dimensional analysis is generally used to check the correctness of derived equations.
Karnataka 1st PUC Physics Chapter 2 Notes
Question 9. How many orders of magnitude greater is the radius of the atom than that of the nucleus?
Answer:
Size of atom = 10-10 m,
Size of atomic nucleus = 10-14 m.
Size of the atom + size of the nucleus is \(\frac{10^{-10}}{10^{-14}}=10^4\)
∴ The size of an atom is 104 times greater than the size of the nucleus.
Question 10. Express unified atomic mass unit in kg.
Answer:
By definition,
1 a.m.u. = \(\frac{1}{12} \times \text { mass of an atom of }{ }_6^{12} \mathrm{C}\)
= \(\frac{1}{12} \times \frac{12 \mathrm{~g}}{6.023 \times 10^{23}}\)
= \(1.67 \times 10^{-24} \mathrm{~g}=1.67 \times 10^{-27} \mathrm{~kg}\)
Chapter 2 Units And Measurements Short Answer Questions
Question 1. The vernier scale of an instrument has 50 divisions which coincide with 49 main scale divisions. If each main scale division is 0.5 mm, then using this instrument what would be the minimum inaccuracy in the measurement of distance?
Answer:
Least count of Vernier Callipers = 1 MSD – 1 VSD
∴ L.C. = 1 MSD – \(\frac{49}{50}\) MSD = \(\frac{1}{50}\) MSD
= \(\frac{1}{50}\) x 0.5 = 0.01 m.m
KSEEB Class 11 Physics Units and Measurements Solutions
Question 2. In a system of units, the unit of force is 100N, the unit of length is 10m and the unit of time is 100s. What is the unit of mass in this system?
Answer:
Here, F = MLT-2 = 100 N …….(1) ;
L = 10 m ; T = 100s
∴ From equation (1)
M X (10) X (100)-2 = 100
⇒ M X 10-3 = 100
⇒ M = \(\frac{100}{10_3}\)
⇒ M = 105 kg
Question 3. The distance of a galaxy from Earth is of the. order of 1025 m. Calculate the order of magnitude of the time taken by light to reach us from the galaxy.
Answer:
Size of galaxy = 1025 m,
Velocity of light, c = 3 x 108 ms-1
Time taken by light to reach Earth,
t = \(\frac{d}{c}=\frac{10^{25}}{3 \times 10^8}=\frac{1}{3} \times 10^{17}=0.3333 \times 10^{17}\)
While calculating the order of magnitude we will consider the power of Ten only.
So the order of magnitude of time taken by light to reach Earth from the galaxy is 1017 seconds.
Question 4. The Earth-Moon distance is about 60 Earth radius. What will be the approximate diameter of the Earth as seen from the Moon?
Answer:
Earth-moon distance, D = 60r.
Diameter of earth, b = 2r
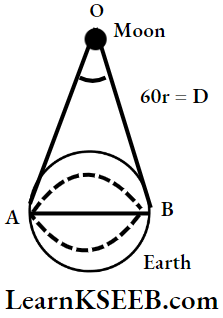
As seen from the moon
⇒ \(\theta=\frac{\text{Arc~} A B}{\text{Rad}}=\frac{2 \mathrm{r}}{60 \mathrm{r}}=\frac{1}{30} \mathrm{Rad}\)
∴ The size of the earth as seen from the moon is \(\frac{1}{30}\)
radians, or \(\frac{57^{\circ} 30^{\prime}}{30} \cong 1.9^{\circ} \text { (or) } 2^{\circ}\)
Question 5. Three measurements of the time for 20 oscillations of a pendulum give t1 = 39.6 s, t2 = 39.9 s, and t3 = 39.5 s. What is the precision of the measurements? What is the accuracy of the measurements?
Answer:
Precision is the least measurable value with that instrument in our case precision is = 0.1 sec.
Calculation Of Accuracy:
Average value of measurements = \(\frac{39.6+39.9+39.5}{3}=\frac{119}{3}=39.67\)
Error in each measurement
⇒ \(\Delta a_1=39.6-39.6=0.07\);
⇒ \(\Delta a_2=39.9-39.67=0.23\);
⇒ \(\Delta a_3=39.67-39.5=0.17\)
∴ \(\Delta a_{\text {mesn }}=\frac{0.07+0.23+0.17}{3}=0.156\)
Precision ± 1 sec. In these measurements only two significant figures are believable. 3rd one is uncertain.
Adjustment of Δamean up to the given significant figure = 0.156 adjusted to 0.2.
So our value is accurate up to ±0.2
So our result is 39.67 ± 0.2, when significant figures are taken into account it is 39.7 ± 0.2 sec.
Question 6. 1 calorie = 4.2 J where 1 J = 1 kg m2s-2. Suppose we employ a system of units in which the unit of mass is α kg, the unit of length is β m, and the unit of time γ s, showing that a calorie has a magnitude 4.2 α-1 β-2 γ2 in the new system.
Answer:
Here, 1 calorie = 4.2 J = 4.2 kg m2/s2 ……(1)
As a new unit of mass = α kg
∴ 1 kg = \(\frac{1}{\alpha}\) new unit of mass
⇒ α-1 new unit of mass
Similarly 1 m = β-1 new unit of length and 1 s = γ-1 new unit of time
Putting these values in (1) we get
1 calorie = 4.2 (α-1 new unit of mass) (β-1 new unit of length)² (γ-1 new unit of time)-2
= 4.2 α-1β-2γ2 new unit of energy, which was proved.
Question 7. A new unit of length is chosen so that the speed of light in vacuum is 1 ms-2. If light takes 8 min and 20s to cover this distance, what is the distance between the Sun and Earth in terms of the new unit?
Answer:
Given the velocity of light in a vacuum,
c = 1 new unit of length s-1
Time taken by the light of the Sun to reach Earth,
t = 8 min 20s = 8 x 60 + 20 = 500 s
∴ Distance between the Sun and Earth, x = c x t
= 1 new unit of length s-1 x 500s
= 500 new units of length.
Question 8. A student measures the thickness of a human hair using a magnification 100 microscope. He makes 20 observations and finds that the average thickness (as viewed in the microscope) is 3.5 mm. What is the estimate of the thickness of hair?
Answer:
Magnification, \(\mathrm{m}=\frac{\text { observed width }(\mathrm{y})}{\text { real width }(\mathrm{x})}\)
⇒ x = \(\frac{\mathrm{y}}{\mathrm{m}}=\frac{3.5 \mathrm{~mm}}{100}=0.035 \mathrm{~mm}\)
∴ The thickness of the hair is 0.035 mm
Question 9. A physical quantity X is related to four mea¬surable quantities a, b, c, and d as follows?
X = a²b³C5/2d-2
The percentage error in the measurement of a, b, c, and d are 1%, 2%, 3% and 4% respectively. What is the percentage error in X?
Answer:
Here, X = a²b³C5/2d-2
⇒ \(\frac{\Delta X}{X}= \pm\left[2\left(\frac{\Delta a}{a}\right)+3\left(\frac{\Delta b}{b}\right)+\frac{5}{2}\left(\frac{\Delta c}{c}\right)+2\left(\frac{\Delta d}{d}\right)\right] \)
= \(\pm\left[2(1 \%)+3(2 \%)+\frac{5}{2}(3 \%)+2(4 \%)\right]\)
= ± 423.5 %
The percentage error in X is ± 23.5 %
Question 10. The velocity of a body is given by v = At² + Bt + C. If v and t are expressed in SI what are the units of A, B, and C?
Answer:
From the principle of Homogeneity, the terms At², Bt, and C must have the same dimensional formula of velocity V.
v = \(\text { Velocity }=\mathrm{LT}^{-1} \Rightarrow \mathrm{LT}^{-1}=\mathrm{A}\left[\mathrm{T}^2\right]\)
∴ A = \(\frac{\mathrm{LT}^{-1}}{\mathrm{~T}^2}=\mathrm{LT}^{-3}\) So unit of A is m/\(\mathrm{sec}^3\)
∴ \(\mathrm{LT}^{-1}=\mathrm{BT} \Rightarrow \mathrm{B}=\mathrm{LT}^{-2}\) So unit of B is \(\mathrm{m} / \mathrm{sec}^2\)
∴ \(\mathrm{LT}^{-1}=\mathrm{C}\)
So the unit of C is \(\mathrm{m} / \mathrm{sec}\).
Dimensional Formulae Of Physical Quantities

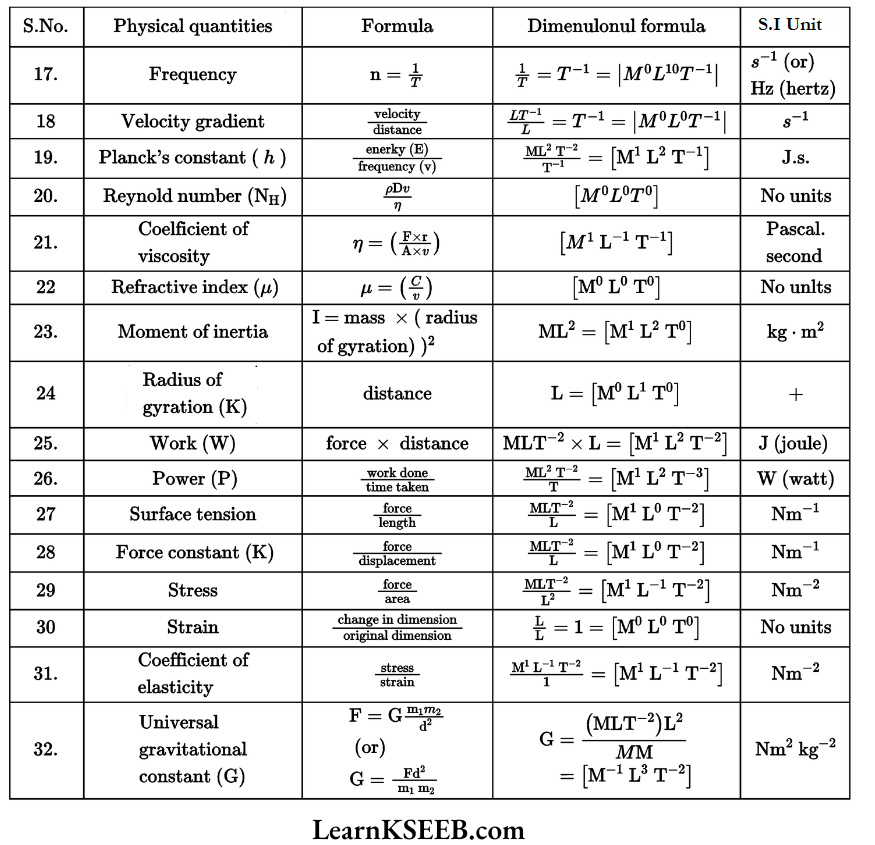
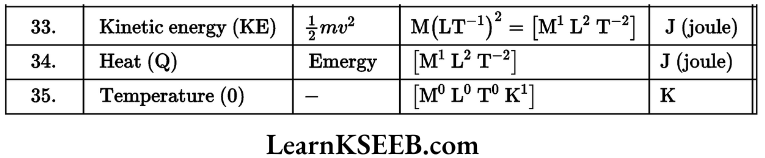
Units And Measurements Problems In KSEEB Physics Chapter 2
Question 1. In the expression, P = El² m-5 G-2 the quantities E, l, m in and G denote energy, angular momentum, mass, and gravitational constant respectively. Show that P is a dimensionless, quantity.
Solution:
Here, P = El² m-5 G-2
Here, I = energy, l = angular momentum
m = mass, G = gravitational constant
= \(\left[M \mathrm{~L}^2 \mathrm{~T}^{-2}\right]\left[\mathrm{M} \mathrm{L}^2 \mathrm{~T}^{-1}\right]^2[\mathrm{M}]^{-5}\left[\mathrm{M}^{-1} \mathrm{~L}^3 \mathrm{~T}^{-2}\right]^{-2}\)
= \(M^{1+2-5+2} \mathrm{~L}^{2+1-6} \mathrm{~T}^{-2-2+4}\)
P = \(\left[\mathrm{M}^0 \mathrm{~L}^0 \mathrm{~T}^0\right]\)
Hence, P is a dimensionless quantity.
Question 2. If the velocity of light c, Planck’s constant, h, and the gravitational constant G are taken as fundamental quantities; then express mass, length, and time in terms of dimensions of these quantities.
Solution:
Here, \(c=\left[L \cdot T^{-1}\right] ; h=\left[M L^2 \mathrm{~T}^{-1}\right]\);
G = \(\left[\mathrm{M}^{-1} \mathrm{~L}^3 \mathrm{~T}^{-2}\right]\)
∴ E = \(\mathrm{h} v, \mathrm{~h}=\frac{\mathrm{E}}{v} ; \mathrm{G}=\left(\frac{\mathrm{Fd}^2}{\mathrm{~m}_1 \mathrm{~m}_2}\right)\)
Let m = \(c^x h^y G^z \rightarrow(1)\)
⇒ \(\left[M^1 L^0 T^0\right]=\left(L^{-1}\right)^x\left(M L^2 T^{-1}\right)^y\left(M^{-1} L^3 T^{-2}\right)^2\)
⇒ \(\left[M^1 L^0 T^0\right]=M^{y-z} L^{x+2 y+3 z} T^{-x-y-2 z}\)
Applying the principle of homogeneity of dimensions, we get
y – z = 1 → (2); x + 2y. + 3z = 0 → (3);
– x – y – 2z = 0 → (4)
Adding equation (2), equation (3) and equation (4),
2y = 1
⇒ y = \(\frac{1}{2}\)
∴ From eq. (2) z = y – 1 = \(\frac{1}{2}\) – 1 = \(\frac{-1}{2}\)
From eq. (4) x = – y – 2z = \(\frac{-1}{2}\) + 1 = \(\frac{1}{2}\)
Substituting the values of x, y, and z in eq. (1), we get
m = \(\mathrm{c}^{1 / 2} \mathrm{~h}^{1 / 2} \mathrm{G}^{-\sqrt{1 / 2}} \Rightarrow \mathrm{m}=\sqrt{\frac{\mathrm{ch}}{\mathrm{G}}}\)
Proceeding as above we can show that
L = \(\sqrt{\frac{\mathrm{hG}}{c^3}}\) and \(\mathrm{T}=\sqrt{\frac{\mathrm{hG}}{\mathrm{c}^5}}\)
Question 3. An artificial satellite revolves around a planet of mass M and radius R, in a circular orbit of radius r. Using dimensional analysis shows that the period of the satellite.
T = \(=\frac{k}{R} \sqrt{\frac{r^3}{g}}\)
where k is a dimensionless constant and g is the acceleration due to gravity.
Solution:
Given that T² ∝ r³ or T ∝ r3/2
Also, T is a function of g and R
Let T ∝ r3/2 ga Rb where a, b are the dimensions of g and R.
(or) T = k r3/2 ga Rb → (1)
where k is a dimensionless constant of proportionality
From equation (1) \(\left[M^0 L^0 T^1\right]=L^{3 / 2}\left(L T^{-2}\right)^a(L)^b=M^0 L^{a+b+\frac{3}{2}} T^{-2 a}\)
Applying the principle of homogeneity of dimensions, we get
a + b + \(\frac{3}{2}=0\)…..(2)
∴ -2 a=1
⇒ a = \(\frac{-1}{2}\)
From eq (1), \(\frac{-1}{2}+b+\frac{3}{2}=0 \Rightarrow b=-1\)
Substituting the values of ‘a’ and ‘b’ in eq. (1), we get
T = \(k r^{3 / 2} \cdot g^{-1 / 2} R^{-1}\)
T = \(\frac{k}{R} \sqrt{\frac{r^3}{g}}\)
This is the required relation.
KSEEB 1st PUC Physics Chapter 2 Important Questions
Question 4. State the number of significant figures in the following
- 6729
- 0.024
- 0.08240
- 6.032
- 4.57 x 108
Solution:
- In 6729 all are significant figures.
- A number of significant figures are Four.
- In 0.024 the zeroes to the left of 1st non-zero digit of a number less than one are not significant.
- A number of significant figures Two.
- 0.08240 – Significant figures Four.
- In 6.032 the zero between two non-zero digits is significant.
- So, a number of significant figures in 6.023 are 4.
- 4.57 x 108 Significant figures Three.
[In the representation of powers of Ten our rule is only significant figures must be given].
Question 5. One stick has a length of 12.132 cm and another has a length of 12.4 cm. If the two sticks are placed end what is the total length? If the two sticks are placed side by side, what Is the difference in their lengths?
Solution:
1. When placed end to end total length is
l = l1 + l2
l1 = 12.132 cm and l2 = 12.4 cm.
∴ l1 + l2 = 12.132 + 12.4 = 24.532 cm.
In addition, the final answer must have the least number of significant numbers in that addition, i.e., one after the decimal point.
So our answer is 24.5 cm.
2. For difference use l1 – l2
i.e., 12.4- 12.132 = 0.268 cm.
In subtraction final answer must be adjusted to the least number of significance figures in that operation.
Here least number is one digit after I decimal. By applying the round-off procedure] our answer is 0.3 cm.
Question 6. Each side of a cube is measured to be 7.203 m. What is
- The total surface area and
- The volume of the cube, to appropriate significant figures?
Solution:
Side of the cube, a = 7.203 m.
So a number of significant figures is Four.
1. Surface area of cube = 6a²
= 6x 7.203x 7.203 = 311.2991
But our final answer must be rounded to the least number of significant figures is four digits.
So the surface area of the cube = 311.3 m³
2. Volume of cube, V = a³ = (7.203)³ = 373.1471
But the answer must be limited to Four! significant figures.
∴ The volume of the sphere, V = 373.1 m³.
Karnataka Board Class 11 Physics Chapter 2 MCQs
Question 7. The measured mass and volume of a body are 2.42 g and 4.7 cm³ respectively with possible errors 0.01 g and 0.1 cm³. Find the maximum error in density.
Solution:
Mass, m = 2.42 g ; Error, Δm = 0.01 g. Volume, V = 4.7 cm³, Error, ΔV = 0.1 cc.
% error in mass = \(\frac{\Delta \mathrm{m}}{\mathrm{m}} \times 100=\frac{0.01}{2.42} \times 100\)
= \(\frac{1}{2.42}\)
% error in volume = \(\frac{0.1}{4.7} \times 100\) = \(\frac{10}{4.7}\)
Density = \(\frac{\text { Mass }}{\text { Volume }}=\frac{M}{V}\)
Maximum % error in density = % error in mass + % error in volume
∴ Maximum percentage error in density = \(\frac{1}{2.42}+\frac{10}{4.7}=0.413+2.127=2.54 \%\)
Question 8. The error in the measurement of the radius of a sphere is 1%. What is the error in the measurement of volume?
Solution:
Percentages Errors in radius = 1% = \(\frac{\Delta r}{r} \times 100\)
The volume of sphere V ∝ r³
⇒ ΔV = 3r²Δr
⇒ \(\frac{\Delta V}{V}=3 \frac{r^2 \cdot \Delta r}{r}\)
Percentage error in volume \(\frac{\Delta \mathrm{V}}{\mathrm{V}}=3\left(\frac{\Delta \mathrm{r}}{\mathrm{r}} \times 100\right)=3 \times 1=3 \%\)
Question 9. The percentage error in the mass and speed are 2% and 3% respectively. What is the maximum error in kinetic energy calculated using these quantities?
Solution:
Percentage change in mass = \(\frac{\Delta m}{m} \times 100\) = 2%
Percentages chage in speed = \(\frac{\Delta v}{v} \times 100\) = 3%
But K.E = \(\frac{1}{2}\) = mv
Percentages change in K.E = 1(% change mass) + 2(% change in velocity)
∴ Percentage change in K.E
= \(1\left(\frac{\Delta \mathrm{m}}{\mathrm{m}} \times 100\right)+2\left(\frac{\Delta \mathrm{v}}{\mathrm{v}} \times 100\right)\)
= 1 x 2 + 2 x 3 = 8%
Class 11 Physics Units and Measurements KSEEB Guide
Question 10. One mole of an ideal gas at standard temperature and pressure occupies 22.4 L (molar volume). If the size of the hydrogen molecule is about 1Å, what is the ratio of molar volume to the atomic volume of a mole of hydrogen?
Solution:
Size of Hydrogen atom = 1Å =10-10 m = 10-8 cm
V1 = Atomic volume = number of atoms x volume of atom.
One mole gas contains ‘n’ molecules.
Avogadro Number, n = 6.022 x 1023
∴ \(V_1=\frac{4}{3} \pi r^3 \times n\)
= \(\frac{4}{3} \times \frac{22}{7} \times\left[10^{-8}\right]^3 \times 6.022 \times 10^{23}\)
= \(25.23 \times 10^{-1}\)or \(2.523 \mathrm{cc} \text {. }\)
∴ \(\mathrm{V}_2\) = Molar volume of 1 mol gas = 22.4 lit
= \(2.24 \times 10^4 \mathrm{c} . \mathrm{c}\)
∴ 1 lit =1000 c.c.
∴ The ratio of molar volume to atomic volume
= \(\mathrm{V}_2: \mathrm{V}_1\)
= \(2.24 \times 10^4: 2.523=10^4 \mathrm{~m}\)
