KSEEB Class 11 Chemistry Notes For Water Pollution
In nature, water is an indispensable component. 97% of the In the nature, water is an indispensable component—97% of the water, which is practically of no use to human beings.
- The remaining 3% is sweet water, of which 2% remains in condensed form in polar regions and in various permanent glaciers.
- The remaining 1% of sweet water, which is accumulated in rivers, fountains, lakes, ponds, under the soil etc., is consumed for different useful purposes.
- Due to the polar nature of water, a large number of inorganic salts get dissolved in it. As a result, these salts become easily available to living beings.
- These salts are extremely important to aquatic life.
- The various gaseous pollutants such as CO2, SOx, NOx etc., present in the atmosphere and organic and inorganic pollutants on the earth’s surface are swept by rainwater and mix with the rivers, lakes, seas etc.
- Due to different chemical reactions in water, these pollutants decompose to give unpolluted water. As a result, the extent of environmental pollution decreases.

Read and Learn More KSEEB Class 11 Chemistry Notes
Water pollution:
When the water of different water bodies gets contaminated with one or more chemical substances, evolved either by natural phenomena or indiscriminate human activities and tend to cause health hazards to man and other living beings or adversely affect the processes of their livelihood, then the water is said to be polluted.
1. Domestic wastes
Solid waste of various materials of domestic use
For example: Discarded paper, plastics, torn cloth, vegetable refuse, remains of food etc.),
Excreta of man and domestic animals are mostly left in open places. With time, these discarded materials are carried by wind or rainwater to the nearby water bodies. This contaminates the water and makes it unfit for use.
Water Pollution Notes for Class 11 KSEEB Board
Water pollution caused by domestic wastes:
1. Domestic wastes mostly contain organic compounds. These organic compounds are decomposed by bacteria in the presence of dissolved oxygen (DO). This process is called biodegradation.
In the process of biodegradation, carbon, hydrogen, nitrogen, phosphorus etc., present in the organic compounds are oxidised to CO2, H2O, nitrate, phosphate and other salts. During the process the quantity of dissolved oxygen gradually decreases. Naturally, aquatic plants, fi hes and other aquatic organisms do not get sufficient oxygen for respiration.
Consequently, aquatic living beings face serious problems. DO is considered to be an important parameter in predicting the quality of water. For aquatic plants and animals, the value of DO must not be less than of 4-6mg L-1 . With the increasing value of DO, the quality of the water gradually improves. Lowering the value of DO indicates that the water is getting polluted.
Biochemical Oxygen Demand or BOD:
Biochemical oxygen demand (BOD) may be defined as the number of milligrams of oxygen required for biodegradation (i.e., biochemical degradation) of organic matter present per litre of polluted water.
- For the determination of the BOD of a sample of water, the sample of water, kept at 20°C, is saturated with oxygen & is subjected to biodegradation (i.e., oxidation) of organic compounds for 5 days by the bacteria present in that water.
- The statement, the BOD of a sample of water is 60 or 60 mg.L-1 means for the biochemical decomposition of organic matter present per litre of water, 60mg of oxygen is required. BOD of the sample of water, if expressed in ppm (parts per million), also gives the same value.
- Greater the value of BOD of water, the higher will be the extent of pollution of that water because if the water contains a large amount of organic matter, the quantity of oxygen required will be high.
- If the value of BOD of a certain sample of water is greater than 5 ppm then, the water is considered to be impure.
Chemical Oxygen Demand or COD :
- Water may sometimes contain organic or inorganic pollutants which are not decomposed by bacteria. These are called nonbiodegradable pollutants.
- So, the value of the BOD of any sample of polluted water does not truly reflect the extent of pollution of that sample of water. Thus, for the determination of the total quantity of biodegradable and non-biodegradable pollutants in a sample of water, the sample of water is oxidised by a strong oxidising agent (K2Cr2O7+ H2SO4) in the laboratory.
- In this case, the oxidising agents supply the necessary oxygen required for the complete oxidation of the pollutants.
- The total amount of oxygen required for the complete oxidation of biodegradable and non-biodegradable pollutants is called Chemical Oxygen Demand (COD). Naturally, the value of COD of any sample of water is always greater than that of BOD.
2. If dissolved oxygen is deficient in water the oxidation of organic pollutants does not get completed. As a consequence of incomplete oxidation, methane (CH4), hydrogen sulphide (H2S), phosphine (PH3), different amino compounds etc., are formed which creates an extremely off ensive odour.
3. Waste materials, sewage from dispensaries, hospitals and domestic wastes carrying pathogenic microorganisms are drained into the water bodies which may result in various diseases such as cholera, typhoid, paratyphoid, dysentery, hepatitis, polio, gastroenteritis, jaundice etc.
4. Waste materials like plastics do not undergo bacterial decomposition in the presence of oxygen, i.e., they are nonbiodegradable. They remain unaffected even if they areleft in water for years. Thus, they decrease the depth of water as well as increase the extent of water pollution under the influence of their constituent chemical compounds
KSEEB Chemistry Class 11 Chapter on Water Pollution
2. Industrial wastes
- Industries release wastes, due to the production of organic and inorganic materials. Factories producing or using mineral acids like HCl, H2SO4, HNO3, H3PO4etc., and alkalis
- For example: NaOH, KOH, and NH3 give up profuse quantities of waste materials or effluents which are thrown directly into the water of rivers, lakes, ponds etc.
- These acids or alkalis get mixed with water and increase the acidity or alkalinity of water.
- Again, the industrial wastes of different factories, containing metallic elements
- For example: Pb, Hg, Cd, Zn, Cr, Mn, As, Be etc.) mixes with different water bodies
- These metals have profound ill effects on aquatic plants and animals in particular.
- Direct use of this polluted water entails attack by several diseases.
- Besides, these metals, accumulated in human bodies through food chains, cause a wide range of diseases
1. Cadmium (cd):
Refining of zinc, copper and lead, electro¬ plating industries, iron and steel factories, Ni-Cd battery factories etc., release cadmium as waste material into rivers and other water reservoirs.
Harmful effects:
Cadmium, introduced into the body through water pollution causes vomiting, irritation of the lungs, malfunctioning of the liver and kidney high blood pressure, anaemia, disorder of bone marrow etc.
Ital-llai disease:
- In 1970, a disease caused by pollution due to cadmium occurred in Toyama Japan and came to be known as Itnl-Itai. The water containing cadmium discharged from a zinc extraction factory situated in that locality was used for irrigation.
- As a result, cadmium was incorporated into rice because of the cultivation of paddy with this polluted water.
- Cadmium was thus introduced into human bodies through this rice when consumed as food. Thus the disease Itai-Itai originated. Pain in bone and joints, weakening or brittleness Of the bone etc., are the symptoms of this disease.
2. Mercury (Hg):
The water discharged as industrial waste from the factories producing caustic soda, chlorine, pesticides etc., is die source of mercury pollution.
Harmful effects:
Mercury is highly toxic. It causes stomach pain, dropsy (oedema), headache etc. Moreover affects the nervous system and kidneys, decreases the reproductive power of males, and babies are found to be born with deformity.
Minamata disease:
In 1953-69, die disease caused by pollution due to mercury appeared in the Minamata area on the sea-coast of Japan and came to be known as Minamata disease. In that area, more than 100 people died of this disease and thousands of people became crippled. In this coastal region, waste materials contaminated with mercury from a polyvinyl factory were regularly discharged into sea¬ water.
Mercury present in the effluent was converted into highly poisonous methyl mercury by different reactions. This poisonous compound was introduced into human bodies through sea fishes and led to the outbreak of Minamata disease. The primary symptoms of this disease are the lack of sensation in muscles, lips, tongue etc., which culminate in blindness and loss of memory
3. Lead (Pb):
The waste materials discharged from factories For example: extraction and refining of lead, paints, varnishes, alloys, batteries and ship-building etc.), containing lead, pollute the water of rivers, lakes and other sources. Apart from these, the anti-knocking compound [Pb(C2H5)4] used in gasoline and petrol is a potential source of lead
Harmful effects:
If water contaminated with lead enters the body, lead gets accumulates in the body. Most of the lead is ultimately deposited in the bone. Lead poisoning gives rise to symptoms such as loss of appetite, vomiting, constipation, anaemia, insomnia, headache etc., and also affects the digestive system.
4. Manganese (Mn):
Effluents containing Mn from ferromanganese producing industries, welding factories and MnO2 as waste materials released from dry batteries, mix with water as pollutants.
Detailed Notes on Water Pollution for KSEEB Class 11
Harmful effects:
If Mn-containing water is consumed for a prolonged time, it causes nervous disorder.
5. Coball (Co):
Industrial discharge from ceramic, paint or dye industries results in the Co-pollution of water.
Harmful effects: If cobalt-contaminated water is consumed, symptoms such as lowering of blood pressure, diarrhoea, deformation of bones etc., are developed.
6. Arsenic (As):
The main sources of arsenic poisoning are pesticides, chemical wastes, pharmaceutical industries, mining by-products etc. In the tube well water in some places, arsenic compounds are present.
Harmful effects:
Water polluted by arsenic disturbs blood circulation in the skin and black or ash spots appear on the skin of the throat, neck and back. The skin of the hands and legs becomes rough and spots like moles appear on the skin. Continuous use of water containing arsenic for a longer time causes cirrhosis of the liver, cancer in the lungs and urinary track
7. Arsenic pollution:
According to the recommendations of the World Health Organisation (WHO), water containing 0.01 mg of arsenic per litre is quite safe for drinking. The limit of arsenic in water that human bodies can sustain is 0.05 mg L-1. But the average arsenic content in the tube well water of some places in the vast region of Bangladesh including some districts of Gangetic West Bengal
For example:
North and South 24-Parganas, Nadia, Murshidabad, Maldah etc.) is 0.25mg. L-1 . As a result of the indiscriminate use of this water, nearly ten lakhs of people have been victimised in West Bengal. Out of these, at least two lakhs of people have been suffering from acute skin diseases
8. Black-foot disease:
Consumption of arsenic contaminated for a long period also causes severe damage to. lower limbs and formation of black lumps on palm and foots. This is known as ‘black-foot disease.
3. Fertilisers used in agriculture
Chemical fertilisers or nutrients are extensively used for increasing the agricultural production. Mainly urea or organic fertilizers and ammonium sulphate, ammonium nitrate, monocalcium phosphate etc., are used as inorganic fertilisers. A certain portion of these fertilisers remains unutilised and being carried by the rainwater, falls into the nearby, lakes etc., and thus causes water pollution
Water containing nitrate ions cannot be used as potable water because nitrate ion cannot be removed by the usual process of purification of water. Consumption of such water affects the haemoglobin of babies, causing the disease called ‘blue baby syndrome’. Moreover, nitrate ions are converted to carcinogenic nitrosamines inside the body
Eutrophication:
- Inorganic fertilizers or nutrients
- For example: Nitrates, phosphates, sulphates etc.)
- Left unutilised in agricultural production, mix with water as waste materials and act as pollutants of the water.
- But, these materials enrich that water with nutrients and help in the rapid growth population of the aquatic plants.
- This higher rate of growth is found to be remarkably high in the case of algae.
- This phenomenon of enrichment of water mixed with fertilizers, causing rapid growth of the population of aquatic plants is called eutrophication.
The ill effects of this over-nutrition i.e., eutrophication may be summarised as:
- The aquatic plants, because of their rapid growth require abundant quantity of oxygen and cause depletion of dissolved oxygen (DO), thereby threatening the survival of aquatic life.
- When the quantity of dissolved oxygen decreases, the anaerobic bacteria grow abnormally and these bacteria react with those waste materials to form different gaseous substances such as methane, ammonia, hydrogen sulphide etc. As aresult, foul smell is emitted.
- With time when the plants die, the remains of the dead plants get deposited at the bottom of lakes, ponds etc., and become shallow.
- In extreme instances of eutrophication, when the population of plants explodes, they exhaust almost the whole of the dissolved oxygen. Consequently, fishes, insects and other aquatic animals die due to the absence of oxygen.
KSEEB Class 11 Environmental Chemistry Water Pollution Notes
4. Pesticides used in agriculture
A wide range of synthetic organic chemicals are used for the better production and preservation of crops. For example, insecticides, fungicides, herbicides etc., are applied to the field to kill insects, fungi, herbs etc. These chemicals are collectively known as pesticides. Pesticides, when used in agricultural fields, are carried by flowing water. Thus, they enter the hydrosphere and cause pollution of water. Again, when pesticides are sprayed in the field, a part of them get mixed with the atmosphere which come down along with rain water and mixes with the water of the rivers, lakes etc. Water pollution is also caused by the wastes discharged from the factories producing pesticides.
Different classes of pesticides and their harmful effects:
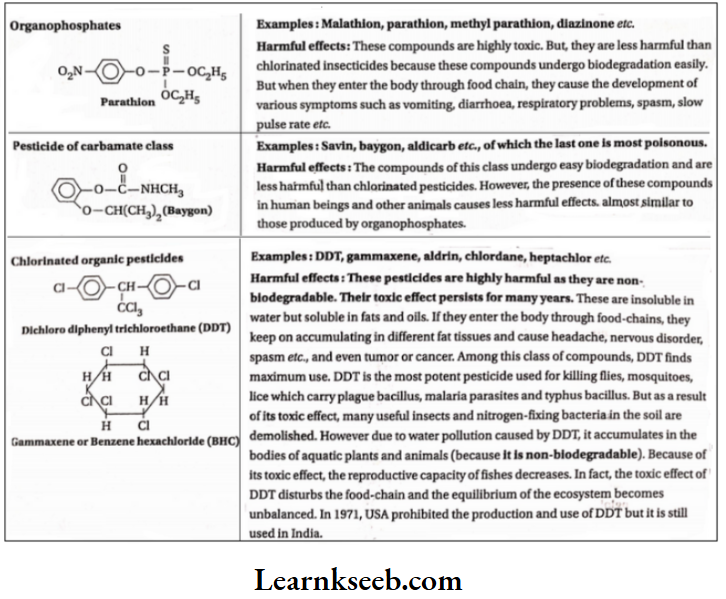
Biomagnification:
There are some pollutants which do no There are some pollutants which do no aldrin, heptachlor etc. These compounds exist for years together, keeping their poisonous effect Intact. These are called permanent organic pollutants.
They are not soluble in water but soluble in fats and oils. So they dissolve in body fats and go on accumulating. These highly toxic substances accumulated in living bodies are transmitted to the bodies of other living beings through food chains. These persistent organic pollutants (POP) exist at highly toxic levels in the bodies of living beings owing to repeated consumption of polluted food
5. Detergent
Detergent is widely used as a cleaning agent in household work and in industry. The effluent released after its use mixes with the nearby ponds, rivers etc., and causes water pollution. Two chief constituents of detergent are—
- Surface active agent: For example: Alkylbenzene sulphonate (ABS).
- Builder of filler: For example sodium Yripolyphosphate [Na5P3O10],if>Both these constituents are responsible for water pollution
Water pollution caused by surface active agents:
1. Surface active agents decrease the surface tension of water and consequently help in the formation of foam emulsion and oily substances with water. These surface active agents are non-biodegradable and thereby entail water pollution.
2. Foam created by detergents forms a layer on the surface of water and thus prevents water from coming in contact with air and sun rays. Consequently, water cannot absorb oxygen from air and the dissolved oxygen level (DO) in water decreases.
3. Furthermore, sun rays being obstructed, the aquatic plants at the bottom cannot release oxygen by the process of photosynthesis. For this reason, also, the dissolved oxygen level gradually gets diminished. This results in the deficiency of oxygen required for the respiration of aquatic plants and animals.
4.. Surface active agents form a layer on some organic pollutants
For example Phenolic compounds
So, phenolic compounds present in water can no longer come in contact with bacteria and hence the biodegradation of organic pollutants becomes inhibited. Consequently, the extent of pollution in water increases.
Pollution caused by builders or fillers
Detergent contains phosphate salts known as builders or fillers. Phosphate ions produced from them form water-soluble complexes by combination with the basic radicals Ca+2, Mg+2 etc. Iff These complex phosphate salts serve as nutrients for algae and aquatic plants, consequently affecting their rapid population growth (Eutrophication). Plenty of oxygen is required for their respiration. This results in rapid decrease in the level of dissolved oxygen (DO) and the survival of aquatic animals becomes extremely difficult.
6. Radioactive substances
Radioactive substances, during mining and refining as well as from nuclear power plants, may be carried into water. Radioactive discharges from medical and scientific institutions using radioactive isotopes may also lead to water pollution.
Harmful effects:
The presence of radioactive substances in trace amounts may cause nervous debility, physical deformity, miscarriage, sterility, cancer, blindness etc. The harmful influence of this radioactivity continues from one generation to another.
7. Thermal pollution
In hydroelectric power plants, generally, the water from rivers or lakes is converted into superheated steam which is used to rotate the turbine. Only a negligible fraction of heat carried by steam is transformed into electrical energy and the rest returns to rivers or lakes with the help of water. This process continues, in cyclic order.
As a result, the temperature of water of the river or the lake rises considerably and the dissolved oxygen (DO) level decreases, causing great harm to the aquatic animals, particularly the fishes. In thermal nuclear power plants and many other industries, water is used as a coolant, which is discharged at a high temperature to rivers or lakes resulting in a rise in the temperature of the water. This increased temperature accelerates the faster assimilation of the waste materials, causing the depletion of dissolved oxygen (DO).
Water Pollution and Its Prevention Class 11 KSEEB Notes
8. Oil-slicks on sea-water
Mineral oils and by-products of oil spread into seawater for several reasons. Consequently, a layer of floating oil (oil slicks) on sea-water is formed and the transfer of atmospheric biochemical level oxygen dissolved of into dissolved oxygen decomposes sea-water(DO)oxygen levels is prevented reduced
- Again, from which further water oxygen in and Naturally required hence reduces this brings about a shortfall of oxygen required for respiration aquatic plants and animals and their survival becomes extremely difficult
- Moreover, oil-slicks on seawater do not allow sun rays to Moreover, oil-slicks on seawater do not allow sun rays to photochemical reactions of aquatic plants are hindered and their growth is remarkably inhibited thereby.
- At sea, the oil layer causes the death of birds. The oil floating on the sea penetrates through the feathers and wings of birds and thus their insulation and buoyancy are adversely affected. Consequently, their body temperature decreases and ultimately they die. This phenomenon is called hypothermia
9. Controls of water pollution
- Septic tanks should be used in every house.
- Bathing and washing of clothes in water bodies like ponds, lakes, rivers etc., should be controlled.
- Application of chemical fertilisers and pesticides must be done within a safe limit.
- Water from sewage systems has to be treated properly.
- Effluents from the industries should not be released directly to the water bodies before proper treatment.
- Oil leakages from oil-loaded ships must be stopped to avoid water pollution.
