KSEEB Class 11 Chemistry Notes For Some Important Compounds Of Calcium Preparation, Properties, And Uses
1. Calcium oxide (quicklime), CaO
Preparation of calcium oxide:
Calcium oxide or quicklime is prepared commercially by heating limestone at about 1250K temperature in lime kilns.
CaCO3 ⇌ CaO + CO2 ↑- 425 kcal
KSEEB Class 11 Chemistry Notes on Calcium Compounds
The reaction is endothermic and reversible. To get a good yield of quicklime, the forward reaction is facilitated by removing CO2 as soon as it is formed. Again, the temperature in the kiln is not allowed to rise above 1270K because above that temperature, silica (SiO2 ) present as
impurity in limestone combines with CaO to form calcium silicate (CaSiO3).
Read and Learn More KSEEB Class 11 Chemistry Notes
CaO + SiO2 >127°K>CaSiO3
Properties of calcium oxide:
1. State: It is a white amorphous solid having a melting point of of2870 K.
2. The action of heat:
Calcium oxide does not melt even when heated in an oxy-hydrogen flame (2270K). On strong heating, it becomes incandescent and emits bright white light (known as limelight). It melts only when heated in an electric furnace at 2850K.
3. The action of air:
When dry lumps of CaO are exposed to moist air, they absorb moisture and CO2 from the air to form calcium hydroxide and calcium carbonate respectively. As a result, heat is evolved and the lumps of CaO are converted into powder. Calcium hydroxide thus produced also reacts with CO2 of air to form calcium carbonate. Therefore, calcium carbonate is the final product we get. However, once the outer surfaces of the lumps of CaO become fully covered with CaCO3, the core material is not further acted upon by moist air.
CaO + H2O-+Ca(OH)2; CaO + CO2→CaCO3
Ca(OH)2 + CO2→ CaCO3 + H2O

Important Calcium Compounds In KSEEB Class 11 Chemistry
4. Reaction with water:
Calcium oxide possesses a high affinity towards water. Many organic liquids and moist gases are dried with CaO. It reacts vigorously with water to form Calcium hydroxide:
CaO + H2O→ Ca(OH)2
When a limited amount of water is sprinkled on the lumps of calcium oxide, a vigorous reaction starts. For its highly exothermic nature, the water added gets immediately transformed into steam with a hissing sound. As a result, the lumps of CaO swell up, crack, and finally crumble to a fine, dry, white powder of calcium hydroxide. Such powdered calcium hydroxide is known as slaked lime and the above process is called the slaking of lime
KSEEB Class 11 Chemistry Important Calcium Compounds Notes PDF
Quicklime when slaked with caustic soda (NaOH), produces a solid called soda lime (CaO + NaOH).
5. Reaction with acids and acidic oxides:
Calcium oxide is a basic oxide and hence reacts with acids to form the corresponding calcium salts and water.
Reaction with acids and acidic oxides Examples:
CaO + 2HCl→CaCl2+ H2O
CaO + H2SO4→CaSO2 + H2O
CaO + 2HNO2→ Ca(NO3)2 + H2O
In the reaction with sulphuric acid, insoluble calcium sulfate is produced and it forms a protective coating on the lumps of CaO. Consequently, the reaction proceeds only to a small extent and then stops.
CaO reacts with acidic oxides to form calcium salts.
Some Important Compounds of Calcium Class 11 Notes KSEEB
Reaction with acids and acidic oxides Examples:
CaO + CO2 → CaCO3 ; CaO + SO2→ CaSO3
6CaO + P4O10 →\(\rightarrow{\Delta}\) 2Ca3(PO4)2
It reacts with silica at a much higher temperature to form calcium silicate: CaO + SiO2→ CaSiO3
6. Reaction with ammonium salts:
Being a strong base, CaO displaces ammonia forming ammonium salts. The reaction occurs rapidly on gentle heating. This reaction may be used for preparing NH3 in the laboratory.
2NH4Cl + CaO→ 2NH3↑ + CaCl2 + H2O
7. Reaction with chlorine gas:
When calcium oxide is heated in the presence of dry Cl2 gas above 573K, calcium chloride is obtained with the evolution of oxygen gas.
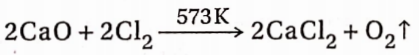
8. Reaction with carbon
When calcium oxide is heated with coke in an electric furnace at about 2273K, it forms calcium carbide and carbon monoxide. This reaction is used in the industrial preparation of calcium carbide.
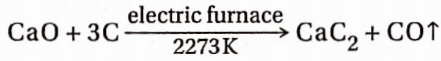
Uses of calcium oxide:
- Quicldime is an important primary material for manufacturing cement and glass.
- It is used to prepare caustic soda from sodium carbonate.
- It is used in the purification of sugar.
- It finds application in the manufacture of dyes.
- It is largely used in the preparation of slaked lime which has many industrial uses.
- It is used as a flux in metallurgy to remove siliceous impurities.
- It is used to dry several gases and alcohols.
- It is used in the preparation of calcium carbide and soda lime.
- It is used in softening of hard water and in tanning industries.
2. Calcium hydroxide or (slaked lime) Ca(OH2)
Preparation of calcium hydroxide:
Calcium hydroxide or slaked lime is prepared by sprinkling a limited amount of water on the lumps of calcium oxide. The process is known as slaking of lime. The reaction is highly exothermic.
CaO + H2O→Ca(OH)2
It can also be prepared by treating a concentrated aqueous calcium chloride solution with a solution of caustic soda.
CaCl2 + 2NaOH→Ca(OH)2↓+ 2NaCl
Class 11 Chemistry KSEEB Calcium Compounds Explanation
Calcium hydroxide Physical properties:
Calcium hydroxide or slaked lime is available as a white amorphous powder. It is soluble in water to a very small extent. The clear dilute aqueous solution of calcium hydroxide is known as lime water. When a large amount of calcium hydroxide is added to water, a white suspension (like milk) of the substance in water is obtained. This is known as milk of lime.
Calcium hydroxide Chemical properties:
1. The action of air: When calcium hydroxide is exposed to air, it slowly absorbs CO2 from the air and is converted into water-insoluble calcium carbonate.
Ca(OH)2 + CO2→ CaCO3 ↓+ H2O
It is to be noted that the formation of a white scum on the surface of clear lime water, exposed to air, is due to the formation of insoluble CaCO3.
2. The action of heat: When calcium hydroxide is heated above 723K, it undergoes complete dehydration to yield CaO.
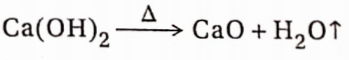
3. Reaction with carbon dioxide:
When carbon dioxide is passed through clear lime water, calcium carbonate is formed. The resultant insoluble calcium carbonate remains suspended in water as fine particles. As a result, clear lime water becomes milky (turbid) in appearance.
Ca(OH)2 + CO2 →CaCO3↓ + H2O
When excess of CO2 gas is passed through this milky suspension, the water-insoluble particles of CaCO3 further react with CO2 in the presence of water to form water-soluble colorless calcium bicarbonate, Ca(HCO3)2. As a result, the turbidity of the solution disappears and it becomes transparent (clear) again.
CaCO3 + CO2 + H2O→ Ca(HCO3)2(soluble)
When tiie clear solution of calcium bicarbonate is heated, the solution again becomes turbid due to the decomposition of Ca(HCO3) into insoluble CnCO3.
4. Reaction with sulfur dioxide:
When SO2 gas is passed through clear lime water, water-insoluble, white calcium sulfite (CaSO3) is formed and the clear solution becomes turbid. When an excess of SO2 gas is passed through this turbid solution, SO2 reacts with CaSO3 in die presence of water to form water-soluble, colourless calcium bisulfite, Ca(HSO3)2. Thus, the turbid solution becomes clear again. When the resultant clear solution is heated, calcium bisulfite decomposes to give back insoluble calcium sulfite along with SO2 and water. So, the clear solution becomes turbid again.
Ca(OH)2 + SO2→ CaSO3↓ + H2O
CaSO3 + SO2 + H2O→Ca(HSO3)2 (soluble)
KSEEB Chemistry Notes for Calcium Carbonate and Calcium Oxide
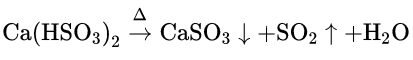
5. Reaction with acid:
Calcium hydroxide being a quite strong base reacts with acids and acidic oxides to form the corresponding salts and water.
Examples:
Ca(OH)2 + 2HCl→CaCl2 + 2H2O
Ca(OH)2 + H2SO4→ CaSO4 ↓+ 2H2O
Ca(OH)2 + CO2 →CaCO3↓- + H2O
Ca(OH)2 + SO2→ CaSO3 + H2O
3Ca(OH)2+ P2O5→ Ca3(PO4)2 + 3H2O
In its reaction with sulphuric acid, insoluble white calcium sulfate is formed and it forms a protective coating on solid Ca(OH)2 and stops the reaction.
Reaction with ammonium salts: Being a stronger base than ammonia, calcium hydroxide displaces ammonia from its salts when heated with an ammonium salt.
Examples: 2NH4Cl + Ca(OH)2→ 2NH3T↑ + CaCl2 + 2H2O
This reaction is generally used for the preparation of ammonia in the laboratory.
6. Reaction with chlorine:
At about 313K, chlorine gas reacts with slightly moist slaked lime to form a dry, white, and powdery substance with a pungent smell. This powder having bleaching and disinfecting properties is commonly called bleaching powder. Regarding the formation and . composition of bleaching powder, the following two views have been proposed.
1. According to Odling (1861), bleaching powder Is a mixture of calcium hypochlorite, Ca(OCl)2, and calcium chloride, CaCl2, and Is called calcium chlorohypochlorite, Ca(OCl). Its formation can be shown as follows
2Ca(OH)2+ 2Cl2 → Ca(OCl)2 + CaCl(Bleaching powder) +2 H2O
Or, 2Ca(OH)2 + 2CI2 → 2Ca(OCl)Cl + 2H2O
Or, Ca(OH)2 + Cl2 → Ca(OCl)Cl(Bleaching powder) + H2O
2. According to Bunn, Clork, and Ghifford (1935), bleaching powder is supposed to be a mixture of
Ca(OCl)2, CaCl2 and Ca(OH)2. Its formation can be represented as
2Cl2 + 3Ca(OH)2→ Ca(OCl)2-Ca(OH)2-CaCl2-2H2O (Bleaching powder)
Wlien Cl2 gas is passed through excess of cold lime water, calcium chloride, and calcium hypochlorite are formed.
2Ca(OH)2 + 2CI2→ CaCl2 + Ca(OCl)2 + 2H2O
When excess chlorine is passed through hot lime water calcium chloride and calcium chlorate are formed.
6Ca(OH)2 + 6Cl2→ 5CaCl2 + Ca(ClO3)2 + 6H2O
Uses of calcium hydroxide
- Calcium hydroxide is used in the manufacture of caustic soda, bleaching powder, superphosphate of lime (a chemical fertilizer), etc.
- It is used in the preparation of mortar, a building material.
- Slaked lime is mixed with three to four times of the weight of sand.
- The mixture is made into a thick paste with a gradual addition of water.
- The paste is called mortar. As the paste becomes dry, it hardens due to the formation of
CaCO3: Ca(OH)2 + CO2→ CaCO3 + H2O
- It is used in the manufacture of glass and in the manufacture of calcium hydrogen sulfate, Ca(HSO4)2 which is used in the paper industry.
- It is used in the recovery of ammonia from NH4Cl (a by-product of the Solvay process), in coal gas purification, in tanneries for removing hair from hides, and for softening hard water. 0 Milk of lime is used for white washing due to its disinfectant properties.
- Lime water is a laboratory reagent for the detection of carbon dioxide.
3. Calcium carbonate (limestone), CaCO3
Natural occurrence: In nature, calcium carbonate occurs in large quantities as limestone, marble, calcite, chalk, etc. It occurs also in the mineral, dolomite which is the double carbonate of calcium and magnesium (CaCO3-MgCO3). Besides these minerals, CaCO3 occurs in abundance in corals, eggshells, outer covering of oysters, snail’s conch, and in teeth and bones of man and animals.
Class 11 KSEEB Chemistry Study Material on Calcium Compounds
Preparation of calcium carbonate: Calcium carbonate may
Be prepared by passing CO2 through lime water or by adding a solution of Na2CO3 to a solution of CaCl.
1 Ca(0H)2 + CO2 → CaCO3↓+ H2O
CaCl2 + Na2 CO3→ CaCO3↓+ 2NaCl
The precipitate of CaCO3 is known as precipitated chalk.
Calcium carbonate Physical properties:
- It is a white solid.
- It is a stable compound which is almost insoluble in water.
Calcium carbonate Chemical properties:
1. The action of heat:
When heated at a higher temperature ( ~ 1270K), calcium carbonate decomposes to give calcium oxide (quicklime) & carbon dioxide. The reaction is reversible and endothermic. So, it proceeds towards completion when the reaction is carried out in an open vessel.
CaCO3 ⇌ CaO + CO2 ↑-heat
2. Reaction with dilute acids: It reacts with dilute acids to form the corresponding calcium salts.
CaCO3 + 2HCl → CaCl2 + CO2↑ + H2O
CaCO3 + H2SO4 → CaSO4 + CO2↑ + H2O
CaCO3 + 2HNO3 → Ca(NO3)2 + CO2↑+ H2O
3. Reaction with carbon dioxide:
When CO2 gas is passed through a fine suspension of calcium carbonate in water, the latter slowly dissolves to produce calcium bicarbonate and thus, a clear solution is obtained.
CaCO3 + CO2 + H2O→ Ca(HCO3)2
When the resultant clear solution is heated, calcium bicarbonate decomposes back to insoluble CaCO3. Thus, the clear solution becomes turbid again. ‘
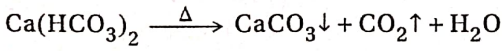
Uses of calcium carbonate:
- Calcium carbonate is largely used in the manufacture of quicklime, cement, and glass.
- Along with MgCO3, it is used as a flux in the extraction of metals such as iron.
- It is used as a building material in the form of marble and the construction of statues.
- Specially precipitated>calcium carbonate is used in the manufacture of high-quality paper.
- Precipitated chalk is used in toothpowder and toothpaste, cosmetics, and also in some medicines (antacids).
4. Plaster of Paris (hemihydrate of calcium sulfate), (CaSO3)2-H2O
Preparation of Plaster of Paris:
Plaster of Paris is prepared by heating gypsum, (CaSO4-2H2O) at about 383-393K in a rotating burner.
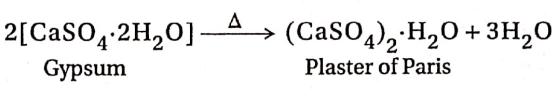
Properties of Plaster of Paris:
- It is a white powder.
- On mixing with an adequate amount of water, it forms a concentrated mixture which solidifies in 5 to 15 minutes due to rehydration. This is called the setting of Plaster of Paris.
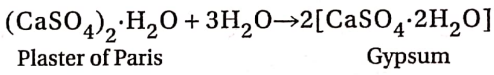
- When Plaster of Paris is heated at about 473K, it forms anhydrous CaSO4. It is called dead burnt plaster and it does not solidify with water. For this reason, during the preparation of Plaster of Paris from gypsum, the temperature should not be allowed to rise above 393K.
Key Compounds Of Calcium In KSEEB Syllabus Class 11 Chemistry
Uses of Plaster of Paris:
- Plaster of Paris is largely used in the building industry.
- It is used in surgical bandages for plastering fractured bones.
- It is used for making casts of statues, molds in pottery work, ornamental castings, and blackboard chalks.
- It is also used in dentistry.
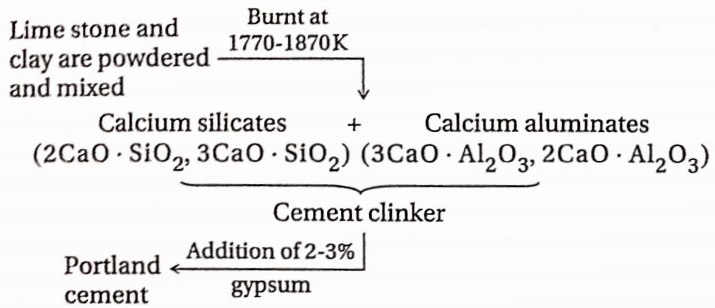
5. Cement
Cement is a mixture of finely powdered calcium silicates and aluminates along with small quantities of gypsum.
The raw materials used for cement are:
- Limestone (CaCO3)
- Clay containing silica (SiO2) and
- Alumina (Al2O3) and
- Gypsum (CaSO4 – 2H2O).
Cement Composition:
Different types of cement have different compositions. The composition of Portland cement is given below:
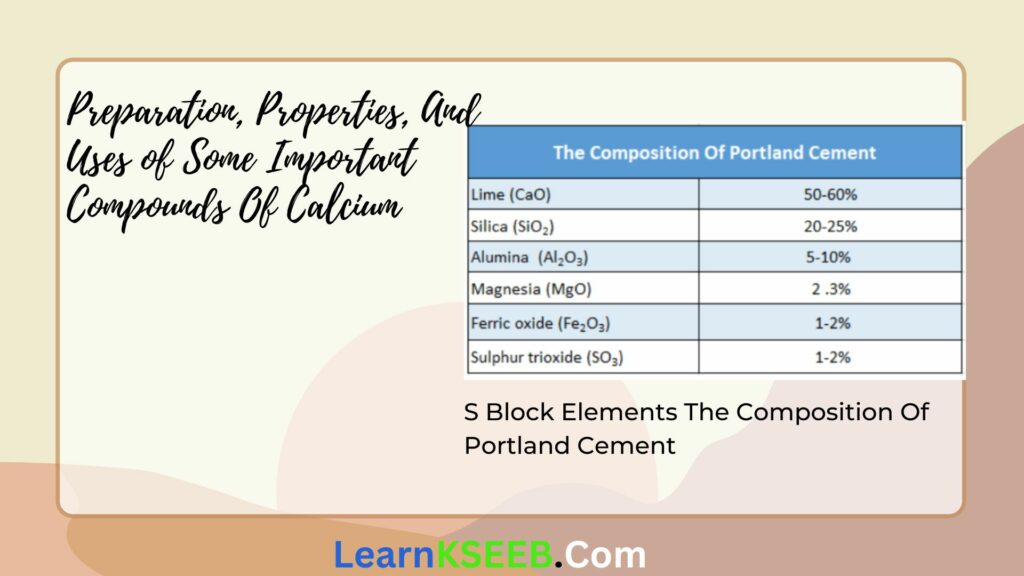
For cement to have good quality, the ratio of silica to alumina should be between 2.5 to 4 and the ratio of CaO to the total oxides of silicon, aluminum, and iron should be close to 2.
Cement Preparation:
For the manufacture of cement, limestone, and clay are fused by strong heating to form cement clinker. This is mixed with the gypsum and ground to a very thin powder.
Cement Settings:
When cement is mixed with water, it forms a plastic mass. After some time it becomes solid. This change is due to the three-dimensional linking between — Si—O — Si — and —Si—O—Al — chains. This transition from plastic to solid is called setting.
Fly ash is a waste product of the steel industry produced mainly due to the burning of coal and carbon compounds. It has similar properties to that of cement. Sometimes fly ash is used with the cement to reduce the cost without compromising on the quality.
Detailed Notes on Important Compounds of Calcium for KSEEB Class 11
Cement Uses:
- Cement is the most important construction material.
- It is used in the construction of tunnels, roads, bridges, etc.
- It is used in concrete and reinforced concrete. These are made by mixing cement with sand, pebbles, and water.
- Mixing with sand it is used for plastering.
Biological Importance Of Magnesium (Mg) And Calcium (Ca)
- Magnesium and calcium ions found in biological fluids play an important role in biological processes. Mg2+ ions are concentrated in cells while C2+ ions are concentrated in body fluids, outside the cell.
- It is known that the energy is stored in the form of ATP. The formations of phosphate linkages are catalyzed by Mg2+ ions. Also, the hydrolysis of phosphate linkages, (Which is accompanied by the release of energy, is also catalyzed by Mg2+ ions.
- Mg2+ ions are present in chlorophyll-a, the green pigment of plants, which absorbs light and is essential for photosynthesis.
- Both these ions are also essential for the transmission of impulses along nerve fibers. About 99% of calcium in the body is present in bones and teeth as apatite, Ca3(PO4)2. In the enamel of teeth, it is present as fluorapatite [3Ca3(PO4)2.CaF2].
- Ca2+ ions also play an important role in blood clotting and are necessary to trigger the contraction of muscles and to maintain regular heartbeats.
- The concentration of Ca2+ ions in blood plasma (about 100mg L-1) is maintained by two hormones namely calcitonin and parathyroid.
- The calcium ions in bones exchange readily with those in blood plasma. About 400 mg of Ca2+ enters and leaves our bones every day.
- In normal adults, there is a balance between this exchange. However, in aged people, especially women, sometimes there occurs a net loss of calcium in the bone leading to a disease called osteoporosis.
- An adult human body contains 1200 g of Ca and 25 g of Mg compared to only 59 g of Fe and 0.06 g of Cu. The daily requirement of calcium and magnesium in the human body has been estimated to be about 200-300 mg. The sources of Mg in our food are nuts, green vegetables, wheat, coffee, etc. while that of Ca are milk, paneer,
