- KSEEB Class 11 Chemistry Notes For Green Chemistry
- KSEEB Class 11 Chemistry Notes For Pressure-Volume Work
- KSEEB Class 11 Chemistry Notes For Soil Pollution
Question 1. In the case of pure water or an aqueous solution, show that pH + pOH =14. Comment on whether this value will be greater than or less than 14 at 0°C and 100°C.
Answer:
We know, that pH+pOH = pKw and pKw= -log
The rise in temperature increases the value of Kw
Therefore, Kw(0 °C) < Kw(25°C)<Kw(100 °C)
Since pKw = -log10Kw,
pKw(100 °C)< pKw(25 °C) < pKw(0°C).
Now, at 25 °C, pKw= 14.
Therefore, pKw (100 °C) < 14 < pKw(0°C)
And pH + pOH(100°C) < pH + pOH(25°C) < pH+ pOH(0°C)
Hence, the value of (pH+ pOH) will be higher at 0°C than at 25 °C, but it will be lower at 100 °C than at 25 °C.
Question 2. At a certain temperature, the Kw of pure water =10-12. VVluit will be the pH -range, and pH of the neutral solution at that temperature.
Answer:
At the given temperature, Kw = 10-12. Hence, at that temperature, pKw= 12. We know that at a particular temperature, the pH -scale ranges from 0 to pKw and pH = \(\frac {1}{2} p K_w\)
For a neutral solution at that temperature. Therefore, at the given temperature, the range of the pH -scale will be from 0 to 12 and the pH of a neutral solution at that temperature will be
⇒ \(\frac {1}{2} p K_w=\frac{1}{2} \times 12=6\)

Short answer questions for Chapter 7 Equilibrium in 1st PUC Chemistry
Question 3. At a certain temperature, the ionization constants of two weak acids, HA and HB are Ka1 and’ ka2, respectively. If Ka1> Ka2 and the concentration of the aqueous solutions of both acids is 0.1(M), then which solution will have a higher pH?
Answer:
As the ionization constant of HA is higher than that of HB, the degree of ionization ofHA in its 0.1 M solution is higher than that of HB in its 0.1 M solution. As a result, the molar concentration of H3O+ions in 0.1(M) HA solution will be more than that in 0.1(M) HB solution. Therefore, the pH of the HB solution will be greater than that of the HA solution.
Question 4. The pH of a buffer solution remains almost unchanged even after dilution—explain.
Answer:
Let a buffer solution that consists of a weak acid (HX) and its salt (MX). For this buffer,
⇒ \(p H=p K_a+\log \frac{[\text { salt }]}{[\text { acid }]}\) At a certain temperature, the value of pKa is constant.
Hence, at a given temperature, the pH of the buffer solution depends upon the ratio of the molar concentrations of the salt to the acid in the solution. As the solution is diluted, this ratio remains the same. As a result, the pH of the solution remains unaltered.
Question 5. Consider the salts given below. For which salt(s) will the pH of the aqueous solution(s) be independent of the concentration of the salt? CH3NH3l, (NH4)3PO4, KCN and (NH4)2CO3.
Answer:
The pH of an aqueous solution of the salt of a weak acid and a weak base does not depend upon the concentration of salt. Among the given salts, (NH4)3PO4 and (NH4)2CO3 are produced from a weak acid and weak base. Hence, the pH of their aqueous solutions does not depend on the concentration of the salt.
Question 6. Write the correct order of increasing acid strength among HCO3< HSO3 < H3O+ < HSO3F.
Answer:
HSO3F is a super acid. So, its strength is the maximum among all the given acids. Comparing the dissociation constants of the remaining acids gives the order in terms of dissociation constant: Now, the larger the value of Ka for an acid, the higher the strength of the acid, So, the increasing order of acid strengths of the given acids will be HCO3< HSO3 < H3O+ < HSO3F.
Question 7. Under experimental conditions/ for the reaction, N2(g) + 3H2(g). 2NH3(g), tithe equilibrium mixture contains 17% of NH3 and 83% of (N2 + H2). Under the same conditions, what will be the percentages of NH3 and (N2 + H2) in the reaction 2NH3(g) N2(g) + 3H2(g) at equilibrium?
Answer:
Under similar experimental conditions, the same equilibrium will be established for a reversible reaction irrespective of whether the reaction is initiated with the reactants or the products. Thus for reaction, 2NH3(g)N2(g)+3H2(g), The same percentages of NH3 and (N2 + H2) will be present at equilibrium as in the case of the reaction N2(g) +3H2(g) 2NH2(g)
1st PUC Chemistry Chapter 7 Equilibrium Important Questions and Answers
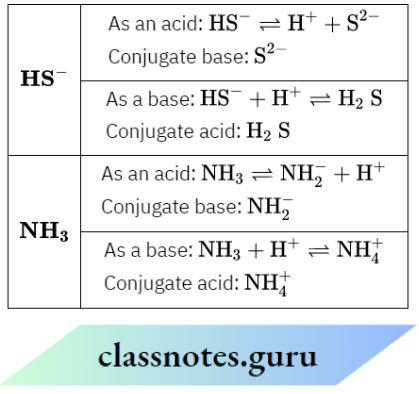
Question 8. Each HS– and NH3 can act as Bronsted acid and Bronsted base—why? Write the formula of conjugate base and conjugate acid in each case.
Answer:
According to the Bronsted-Lowry concept, an acid is a proton donor and a base is a proton acceptor. The given species can accept as well as donate protons, so they can act as both acid and base.
Question 9. The concentration of H3O+ ions in solution is 1000 times that in solution B. What is the difference between the values of pH of these two solutions?
Answer:
Given: [HgO+]a = 1000 × [H3O+]
Where [H2O+]A and [H3O+]B are the concentrations of H3O+ ions in solutions A and B respectively.
Therefore, pH (solution A)
= -log10[H3O+]A = -log(103×[H3O+]B
=- 3- log10[H3O+]B =- 3 + pH of solution B
pH of (solution B) – pH of (solution A) = 3
A chemical reaction, is generally, associated with an absorption or evolution of heat However, many reactions also involve mechanical work along with the absorption or evolution of heat.
This work is done either by the system on its surroundings or by the surroundings of the system. The mechanical work associated with a chemical reaction is due to the change in the volume ofthe reaction system.
If the volume of a system increases against an external pressure or decreases by an external pressure, then some amount of mechanical work is performed because of the change in the volume of the system. This work is called pressure-volume work (or P-Vwork).
Read and Learn More KSEEB Class 11 Chemistry Notes
In a reaction, if one or more gaseous products are formed [example Zn(s) + 2HCl(aq)→ZnCl2(aq) + H2(g) ] or the number of moles of gaseous substances increases [example PCl5(g)→PCl3(g) + Cl2(g) ], then the volume of the system increases against external pressure (usually against atmospheric pressure). The work is thus performed by the system on the surroundings.
On the other hand, in a reaction, if the gaseous reactants are consumed [example 2H2(g) + O2(g) 2H2O(1) ] or the number of moles of gaseous substances decreases [example N2(g) + 3H2(g) 2NH3(g) ], then work is performed on the system! by the surroundings under the influence of external pressure.

KSEEB Class 11 Chemistry Notes For Pressure Volume Work
Calculation of pressure-volume (P-V) work:
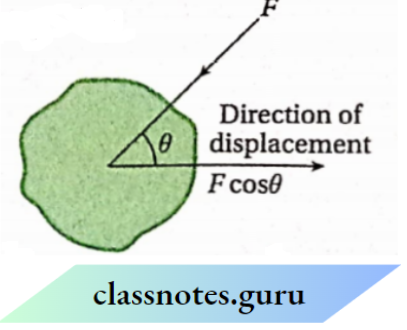
According to mechanics, if dx is the displacement of the point of application of force F acting on a particular body, then [ work done = Fcost? dx [where 6 is the angle between the direction of applied F and the direction of displacement (dx) of the point, FcosB is the component of force along the direction ofdisplacement] If the applied force (F) and f . the displacement (dx) be in the same direction, then. 9 = 0 and cos# = 1 . Hence, in this case, we can write,
The equation for pressure-volume work:
Let us consider, that a gas is kept In a cylinder fitted with a weightless and frictionless piston. The piston Is held up by the stops (s,s).
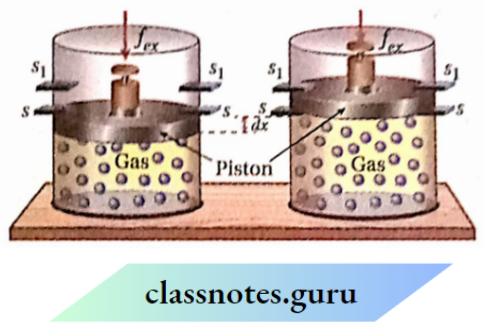
Suppose, an external force is applied to the piston. If the cross-sectional area of the piston is A then external pressure, Pex \(=\frac{f_{e x}}{A}\) Suppose, Pex is less than the pressure of the gas.
Now, if stops (s, s) are removed, the gas will expand against external pressure till the piston is again held up by the stops (s1, S1). The piston moves a distance of dx, then work is done by the system on the surroundings
⇒ \(\delta w=-f_{e x} d x=-P_{e x} A d x\) [since \(f_{e x}=P_{e x} A\)
[According to the convention, work done by the system is -ve ] Now, A dx – dV = increase in volume ofthe gas (system) due to the displacement of the piston. So, \(\delta w=-P_{e x} d V\) Let us consider the gas is expanded from its initial volume K1 to final volume V2. If this expansion is carried out in several steps, then the total work done by the gas.
⇒ \(w=-\int_{V_1}^{V_2} P_{e x} d V\)
[since Since work is not a state function, the value of \(\int_1^2 \delta w\) cannot be expressed as (u/2- u/1, instead we represent it as w .] Equation [1] is the general expression of P-Vwork. Work done by the gas and work done on the gas both can be calculated by using equation [1].
Expansion work of a gat:
If a gas expands from iu initial volume V1 to final volume V2 against a constant eternal pressure of V2, then work done
⇒ \(w=-\int_{V_1}^{V_2} p_{e x} d V=p_{e x}\left(V_2-V_1\right)\left\{p_{s x}=\text { constant }\right]\)
⇒ \(=-P_{e x} \Delta V\)
⇒ \(w=-P_{e x} \Delta V=-v e\)
Since \(V_2>V_1, \Delta V=V_2-V_1=+v e j\)
Conventionally, the Work done by the system is negative.
Pressure-Volume Work Explained In KSEEB Class 11 Chemistry
Compression work of a gas:
If a gas is compressed from its initial volume V1 to final volume V2 where a constant external pressure is Pex, then work done, \(w=-\int_{V_1}^{V_2} P_{e x} d V=-P_{e x}\left(V_2-V_1\right)\)
since Pex constant
⇒ \(=-P_{e x} \Delta V\)
∴ \(w=-P_{e x} \Delta V=+v e\)
Since \(V_2<V_1 \text { and } \Delta V=V_2-V_1=-v e\)
Conventionally, work done on the die system is positive.
Pressure-volume work in a reversible process
A reversible process is completed by an infinite number of small steps. In each step of this process, the driving force is infinitesimally greater than the opposing force.
Let V1 be the volume of a certain amount of a gas confined in a cylinder fitted with a weightless & frictionless piston. The gas is expanded reversibly from volume V1 to V2.
If the external pressure (Pex) on the gas is equal to the pressure ofthe gas (P), then there will be no displacement ofthe piston. Consequently, the volume ofthe gas does not change and the system remains in equilibrium.
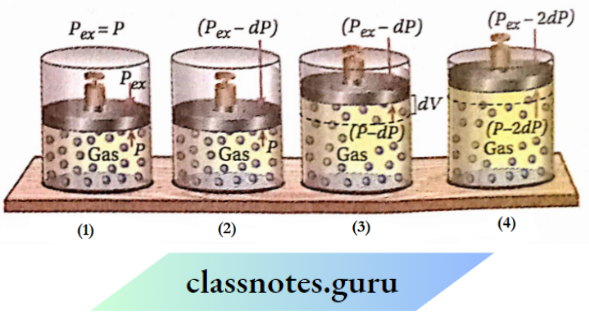
Now, external pressure is diminished by an infinitesimal amount of DP. As a result, the gas will start to expand against pressure (Pex- dP) until the pressure of the gas equals the pressure (Pgx- dP). Suppose the volume is increased by an amount of dV. At the end of this expansion, the system regains its equilibrium state.
Again, external pressure is further decreased by an amount of DP. Consequently, the volume ofthe gas also increases by an infinitesimal amount of dV, and at the end of the expansion, the equilibrium of the system is restored. In this way, the gas is made to expand in an infinite number of small steps until the volume ofthe gas reaches V2.
Expression of pressure-volume work in a reversible process: In a reversible expansion of a gas, in each step, external pressure (Pex) is infinitesimally smaller than the pressure of the gas (P). Thus, in each step, Pgx is considered to be almost equal to P. So, in each step work done, 8wrgv = -PgxdV =-PdV [P = pressure ofthe gas at respective step, dV= infinitesimal increase in volume due to infinitesimal decrease in pressure & ‘rev’ = reversible]. If in a reversible expansion, the volume ofa gas is increased from V1 to V2, then-
⇒ \(w_{r e v}=-\int_{V_1}^{V_2} P d V\)
Equation [1] can be used to calculate the work done by a gas in its reversible expansion. To integrate equation [1], it is necessary to know the variation of pressure of the gas with volume. Work done on the gas in a reversible compression can also be calculated using equation [1], in this case, V2 < V1.
KSEEB Chemistry Notes On Pressure-Volume Work Class 11
Work done by an ideal gas in its isothermal reversible expansion
Let us consider that n mol of an ideal gas is enclosed in a cylinder fitted with a weightless and frictionless piston. The initial pressure, temperature, and volume of the gas are P1, T, and V1 respectively. Now the gas is expanded reversibly from volume V1 to V2 under isothermal conditions. Suppose, due to this expansion, the pressure of the gas decreases from P1 to P2.
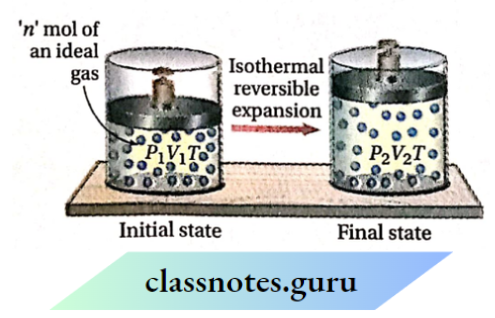
Calculation of work done: Work done due to isothermal reversible expansion,
⇒ \(w_{r e v}=-\int_{V_1}^{V_2} P d V=-\int_{V_1}^{V_2} \frac{n R T}{V} d V\)
∴ \(P=\frac{n R T}{V}\)
As the process is isothermal, so Tremains constant
∴ \(w_{r e v}=-n R T \int_{V_1}^{V_2} \frac{d V}{V}\)
∴ \(w_{r e v}=-n R T \ln \frac{V_2}{V_1}=-2.303 n R T \log \frac{V_2}{V_1}\)
At constant temperature for an ideal gas \(\frac{V_2}{V_1}=\frac{P_1}{P_2}\)
In equations [1] and [2], wrong is negative because during expansion V2 > & P1 > P2. This conforms with the convention because the sign of work done by the system is negative. Therefore, applying equations [1] and [2], it is possible to calculate the amount of work done by an ideal gas (system) in an isothermal reversible expansion.
Work done on an ideal gas in its isothermal reversible compression
Let us consider that n mol of an ideal gas is enclosed in a cylinder fitted with a weightless and frictionless piston. The initial pressure, temperature, and volume (before compression) are Px, T, and V respectively. Now the gas is compressed reversibly from volume V1 to V2 under isothermal conditions.
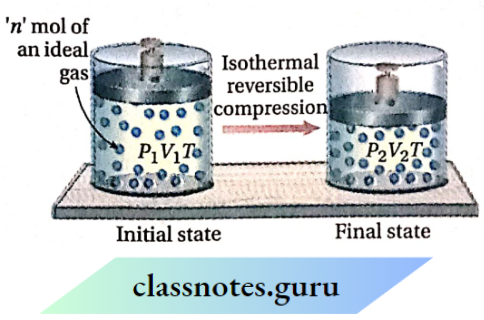
Suppose, the pressure of the gas increases from P1 to P2 because of this compression.
Calculation of work done: Work done in the isothermal reversible compression,
⇒ \(w_{r e v}=-n R T \ln \frac{V_2}{v_1}\) …………………………….(1)
= \(w_{r e v}=-n R T \ln \frac{P_1}{P_2}=-2.303 n R T \log \frac{P_1}{P_2}\) …………………………….(2)
In equations (1) and (2), the sign is positive because V2 < V1 and P2 > P1. This conforms with the convention because the sign of work done on the system is positive. Therefore, applying equations (1) and (2), it is possible to calculate the amount of work done on an ideal gas (system) in its isothermal reversible compression.
Work done by a gas in its irreversible expansion
Let us assume that a certain amount of gas is kept in a cylinder fitted with a weightless and frictionless piston. Suppose, the initial volume and pressure of the gas are V1 and P1 respectively
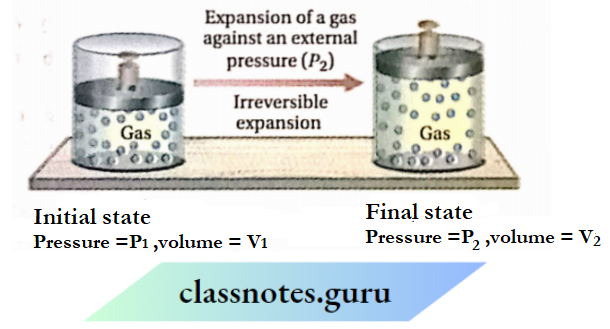
If the external pressure is suddenly reduced to P2 (where P2 is much less than P1 ), the gas will go on expanding till the internal pressure becomes equal to P2. Let the volume of the gas increase from V1 to V2 due to the lowering of pressure from P1 to P2.
Calculation of Work done: Work done by the gas
⇒ \(w_{i r r}=-\int_{V_1}^{V_2} P_{e x} d V=-\int_{V_1}^{V_2} P_2 d V\)
[External pressure Pex= P2Irr = irreversible]
∴ \(w_{i r r}=-P_2\left(V_2-V_1\right)\) ……………..(1)
As \(v_2>v_1, w_{i r r}\) = negative. This agrees with the convention as the sign of work done by the system is negative. The amount of work done by the gas in its irreversible expansion against a constant external pressure can be calculated by using the above equation (1),
KSEEB Class 11 Chemistry Pressure-Volume Work Solutions
Work done by n mol of an Ideal gas in Its isothermal irreversible expansion:
From equation [1] we obtain
⇒ \(w_{i r r}=-P_2\left(V_2-V_1\right) \text {; where, } V_2>V_1 \text {. For } n \mathrm{~mol} \text { of an }\)
⇒ \(\text { ideal gas, } V=\frac{n R T}{P} \text {. At the initial state, } V_1=\frac{n R T}{P_1} \text { and at }\) \(\text { final state, } V_2=\frac{n R T}{P_2}\)
Therefore ⇒ \(w_{i r r}=-P_2\left(\frac{n R T}{P_2}-\frac{n R T}{P_1}\right)\)
∴ \(w_{i r r}=-n R T\left(1-\frac{P_2}{P_1}\right)\left[P_2<P_1\right]\)
The amount of work done by n mol of an ideal gas is due to its isothermal irreversible expansion can be calculated by using equation (1) or (2)
Work done on a gas in its irreversible compression
Let us consider a gas kept in a cylinder fitted with a frictionless and weightless piston is in thermodynamic equilibrium. Suppose, the initial pressure and volume of the gas are P1 and V1 respectively.
As the gas is in the state of thermodynamic equilibrium, the internal and external pressures are equal. If the external pressure is suddenly increased from (P1 to P2 (where P2 is much greater than P1 ), the volume of the gas will keep on decreasing until the internal pressure becomes equal to the external pressure P2. Suppose the volume of the gas decreases from V1 to V2 due to an increase in pressure from P1 to P2.
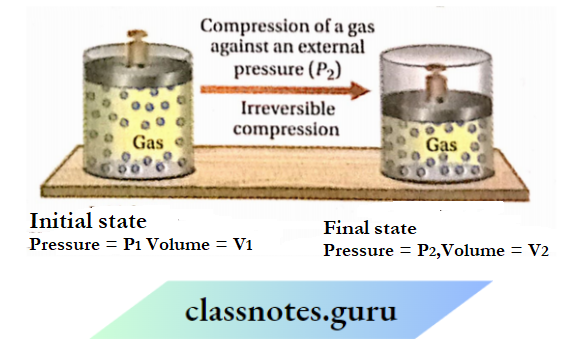
Calculation of work done:
⇒ \(w_{i r r}=-\int_{V_1}^{V_2} P_{e x} d V=-\int_{V_1}^{V_2} P_2 d V\)
External pressure Pex = P2
∴ \(w_{i r r}=-P_2\left(V_2-V_1\right)\) ……………………..(1)
∴ \(w_{i r r}=\text { positive }\left[V_2<V_1\right]\)
This agrees with the convention because the sign of work done on the system is positive.
The amount of work done on the gas (by the surroundings) in an isothermal irreversible compression can be calculated try the equation 11 {. Work done by n mol of an Ideal gnu In Its Isothermal Irreversible compression: Prom the equation |1| we obtain
⇒ \(w_{i r r}=-P_2\left(V_2-V_1\right) \quad\left|V_2<V_1\right|\)
⇒ \(\text { Initial volume, } V_1=\frac{n R T}{P_1} \text { and the final volume } V_2=\frac{n R T}{P_2}\)
Therefore \(w_{i r}=-P_2\left(\frac{n n T}{P_2}-\frac{n R T}{P_1}\right) .\)
∴ \(w_{i r r}=-n R T\left(1-\frac{P_2}{P_1}\right)\left[P_2>P_1\right]\)
The amount of work done on n mol of an ideal gas in its isothermal irreversible compression can be calculated by the equation [1] or [2].
The magnitude of work done by a gas in its reversible expansion is always greater than that in its irreversible expansion provided the initial and final states of the system arc are identical in hodi die cases.
Fit tu on Reversible expansion of gas occurs through a large number of infinitesimal steps, and in each step, the external pressure differs from the pressure of the gas by an infinitesimal amount. Therefore, in each step of a reversible expansion, the gas is expanded against the maximum possible pressure. As a result, The work obtained in a reversible expansion is maximum.
On die other hand, in an irreversible expansion of a gas, the external pressure differs from the pressure of the gas by a finite amount. In this expansion, since the gas expands against an external pressure fairly less than the pressure of the gas, the work obtained is always less than the reversible expansion.
Work done by a gas in its free expansion
In free expansion, the gas expands against zero external pressure. The work done
⇒ \(\boldsymbol{w}=-\int_{V_1}^{V_2} \boldsymbol{P}_{e x} d V=-\int_{V_1}^{V_2} 0 \times d V=0\)
[Since the gas expands against zero pressure, Pex = 0] Therefore, the work done by a gas in its free expansion is zero.
Pressure-volume work in a chemical reaction
At a particular temperature and pressure, the change in volume of a reaction system is considered to be made up of reactants and products involved in the reaction depending primarily on the change in the number of moles of gaseous substances that participated in the reaction.
This is because the change in the number of moles of solid or liquid substances that participated in a reaction has a negligible effect on the volume of the reaction system.
Pressure-volume work in a chemical reaction Example:
When I mol of Zn reacts completely with dilute HCl, 1 mol of H2 gas is produced. In this reaction, the change in volume of the reaction system will be approximately equal to the volume of 1 mol of H2 since the contribution of other constituents towards the volume change is negligible.
Suppose, at constant temperature ( T) and pressure, the difference in volume between the gaseous products and reactants in a reaction is AV’. So. work done, w = -PΔV. If the difference in the number of moles between the gaseous products and the gaseous reactants Is An and these gases behave like an ideal gas, then work done
⇒ \(w=-P \Delta V=-\Delta n R T\)
If Δn > 0, (Example \(\left.\mathrm{Zn}(s)+2 \mathrm{HCl}(a q) \rightarrow \mathrm{ZnCl}_2(a q)+\mathrm{H}_2(g)\right) .\). then w is negative. In this case, work is done by the system on die surroundings.
If Δn < 0 , [Example \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(l)\). then w is positive, in this case, work is done on the system by the surroundings.
A system can interact with its surroundings by exchanging energy either in the form of heat or work or both. The interaction brings about changes in the properties ofthe system
Heat Definition
In thermodynamics, heat is defined as the energy flowing across the boundary of a system by the temperature difference between the system and its surroundings.
Work Definition
In thermodynamics, work is defined as the energy transferred between the system and its surroundings due to the existence of unbalanced forces between the two.
Read and Learn More KSEEB Class 11 Chemistry Notes
Some important features of heat and work:
Transfer of heat, work occurs only at the boundary of a system.Both heat and work are the forms of energy in transit. Heat or work appears only when a system undergoes a process. There is no existence of heat or work before or after the process.
KSEEB Class 11 Chemistry Notes On Thermodynamics
Heat And Work Explanation:
In the following example it can be shown that the work and heat are not the state functions ofa system. Let us consider, that 1 mol ofice at (0°C and 1atm) is to be converted into water at the same temperature and pressure.
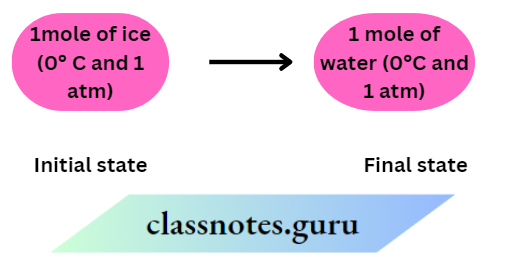
This transformation can be carried out by following the two alternative ways given below.
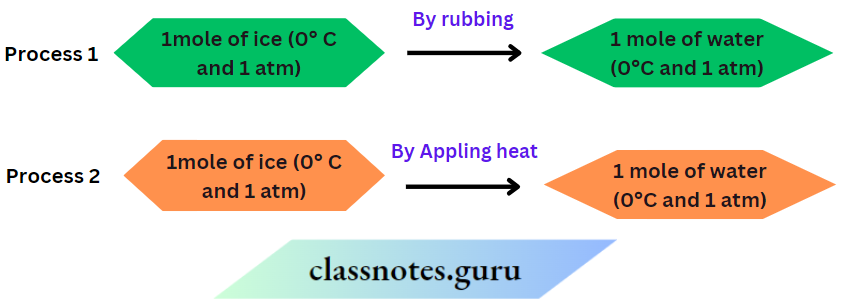
In Work is done during rubbing but no heat is transferred. But in heat is transferred but no work is done. Although the initial and final states of the system are the same in both processes, the amount of work or heat involved in these processes is different. Thus, work done or heat transferred in a process depends on the route followed to carry out the process.
In a process, the change in a property of a system is calculated by subtracting the final value from the initial value. Generally, this difference is denoted by the symbol ‘A’. Heat and work are not the properties of the system. Heat and work are the energies in transit. There is no existence of heat and work before or after a process. Thus, we can write AP or AV, but not Aq or Aw.
Sign of heat and work in thermodynamics:
Sign of heat:
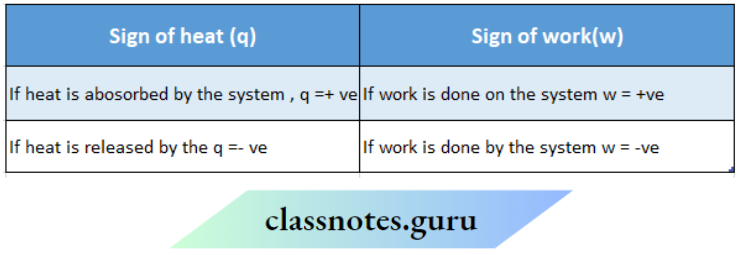
The amount of heat transferred is expressed by Q If heat is transferred from the surroundings to the system, the system gains energy. The gain of heat by the system is represented by the +ve sign. For example, if a system absorbs local heat from the surroundings, then q =+10 cal.
If heat is transferred from the system to the surroundings, the system loses energy. The heat rejected by the system is represented by the -ve sign. For example, if a system rejects 10 cal of heat to the surroundings, then q = -10 cal.
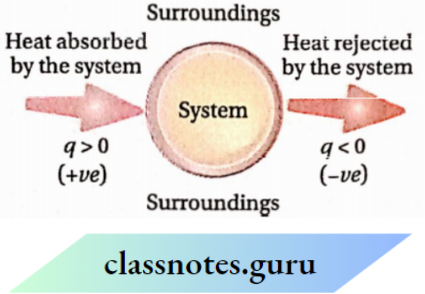
Thermodynamics Notes For KSEEB Class 11 Chemistry
Sign of work:
Work done on the system or by the system is denoted by w. According to the IUPAC convention, the energy ofthe system decreases when work is done by it. So, w is negative. On the other hand, the energy ofthe system increases if work is done on it. So it is positive, Example if 10 kj of work is performed by a system, then w = -10 kl. On the other hand, if 10 kj of work is performed on a system, w = +10 kj
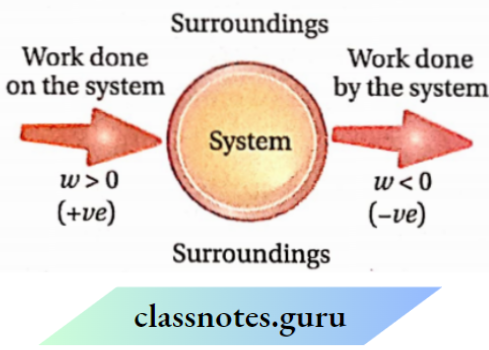
Sign conventions for heat (?) and work (m):
Units of heat and work:
Units of heat: The CGS unit of heat is calorie. The SI unit of heat is joule. [1 cal = 4.184 joule]
Units of work: The traditional unit of work is erg. lerg =1 dyn. cm. SI unit of work is joule. 1 joule =1 N. m = 107 erg.
1. Calcium oxide (quicklime), CaO
Preparation of calcium oxide:
Calcium oxide or quicklime is prepared commercially by heating limestone at about 1250K temperature in lime kilns.
CaCO3 ⇌ CaO + CO2 ↑- 425 kcal
The reaction is endothermic and reversible. To get a good yield of quicklime, the forward reaction is facilitated by removing CO2 as soon as it is formed. Again, the temperature in the kiln is not allowed to rise above 1270K because above that temperature, silica (SiO2 ) present as
impurity in limestone combines with CaO to form calcium silicate (CaSiO3).
Read and Learn More KSEEB Class 11 Chemistry Notes
CaO + SiO2 >127°K>CaSiO3
Properties of calcium oxide:
1. State: It is a white amorphous solid having a melting point of of2870 K.
2. The action of heat:
Calcium oxide does not melt even when heated in an oxy-hydrogen flame (2270K). On strong heating, it becomes incandescent and emits bright white light (known as limelight). It melts only when heated in an electric furnace at 2850K.
3. The action of air:
When dry lumps of CaO are exposed to moist air, they absorb moisture and CO2 from the air to form calcium hydroxide and calcium carbonate respectively. As a result, heat is evolved and the lumps of CaO are converted into powder. Calcium hydroxide thus produced also reacts with CO2 of air to form calcium carbonate. Therefore, calcium carbonate is the final product we get. However, once the outer surfaces of the lumps of CaO become fully covered with CaCO3, the core material is not further acted upon by moist air.
CaO + H2O-+Ca(OH)2; CaO + CO2→CaCO3
Ca(OH)2 + CO2→ CaCO3 + H2O

Important Calcium Compounds In KSEEB Class 11 Chemistry
4. Reaction with water:
Calcium oxide possesses a high affinity towards water. Many organic liquids and moist gases are dried with CaO. It reacts vigorously with water to form Calcium hydroxide:
CaO + H2O→ Ca(OH)2
When a limited amount of water is sprinkled on the lumps of calcium oxide, a vigorous reaction starts. For its highly exothermic nature, the water added gets immediately transformed into steam with a hissing sound. As a result, the lumps of CaO swell up, crack, and finally crumble to a fine, dry, white powder of calcium hydroxide. Such powdered calcium hydroxide is known as slaked lime and the above process is called the slaking of lime
Quicklime when slaked with caustic soda (NaOH), produces a solid called soda lime (CaO + NaOH).
5. Reaction with acids and acidic oxides:
Calcium oxide is a basic oxide and hence reacts with acids to form the corresponding calcium salts and water.
Reaction with acids and acidic oxides Examples:
CaO + 2HCl→CaCl2+ H2O
CaO + H2SO4→CaSO2 + H2O
CaO + 2HNO2→ Ca(NO3)2 + H2O
In the reaction with sulphuric acid, insoluble calcium sulfate is produced and it forms a protective coating on the lumps of CaO. Consequently, the reaction proceeds only to a small extent and then stops.
CaO reacts with acidic oxides to form calcium salts.
Reaction with acids and acidic oxides Examples:
CaO + CO2 → CaCO3 ; CaO + SO2→ CaSO3
6CaO + P4O10 →\(\rightarrow{\Delta}\) 2Ca3(PO4)2
It reacts with silica at a much higher temperature to form calcium silicate: CaO + SiO2→ CaSiO3
6. Reaction with ammonium salts:
Being a strong base, CaO displaces ammonia forming ammonium salts. The reaction occurs rapidly on gentle heating. This reaction may be used for preparing NH3 in the laboratory.
2NH4Cl + CaO→ 2NH3↑ + CaCl2 + H2O
7. Reaction with chlorine gas:
When calcium oxide is heated in the presence of dry Cl2 gas above 573K, calcium chloride is obtained with the evolution of oxygen gas.
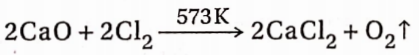
8. Reaction with carbon
When calcium oxide is heated with coke in an electric furnace at about 2273K, it forms calcium carbide and carbon monoxide. This reaction is used in the industrial preparation of calcium carbide.
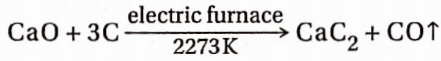
Uses of calcium oxide:
2. Calcium hydroxide or (slaked lime) Ca(OH2)
Preparation of calcium hydroxide:
Calcium hydroxide or slaked lime is prepared by sprinkling a limited amount of water on the lumps of calcium oxide. The process is known as slaking of lime. The reaction is highly exothermic.
CaO + H2O→Ca(OH)2
It can also be prepared by treating a concentrated aqueous calcium chloride solution with a solution of caustic soda.
CaCl2 + 2NaOH→Ca(OH)2↓+ 2NaCl
Calcium hydroxide Physical properties:
Calcium hydroxide or slaked lime is available as a white amorphous powder. It is soluble in water to a very small extent. The clear dilute aqueous solution of calcium hydroxide is known as lime water. When a large amount of calcium hydroxide is added to water, a white suspension (like milk) of the substance in water is obtained. This is known as milk of lime.
Calcium hydroxide Chemical properties:
1. The action of air: When calcium hydroxide is exposed to air, it slowly absorbs CO2 from the air and is converted into water-insoluble calcium carbonate.
Ca(OH)2 + CO2→ CaCO3 ↓+ H2O
It is to be noted that the formation of a white scum on the surface of clear lime water, exposed to air, is due to the formation of insoluble CaCO3.
2. The action of heat: When calcium hydroxide is heated above 723K, it undergoes complete dehydration to yield CaO.
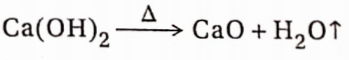
3. Reaction with carbon dioxide:
When carbon dioxide is passed through clear lime water, calcium carbonate is formed. The resultant insoluble calcium carbonate remains suspended in water as fine particles. As a result, clear lime water becomes milky (turbid) in appearance.
Ca(OH)2 + CO2 →CaCO3↓ + H2O
When excess of CO2 gas is passed through this milky suspension, the water-insoluble particles of CaCO3 further react with CO2 in the presence of water to form water-soluble colorless calcium bicarbonate, Ca(HCO3)2. As a result, the turbidity of the solution disappears and it becomes transparent (clear) again.
CaCO3 + CO2 + H2O→ Ca(HCO3)2(soluble)
When tiie clear solution of calcium bicarbonate is heated, the solution again becomes turbid due to the decomposition of Ca(HCO3) into insoluble CnCO3.
4. Reaction with sulfur dioxide:
When SO2 gas is passed through clear lime water, water-insoluble, white calcium sulfite (CaSO3) is formed and the clear solution becomes turbid. When an excess of SO2 gas is passed through this turbid solution, SO2 reacts with CaSO3 in die presence of water to form water-soluble, colourless calcium bisulfite, Ca(HSO3)2. Thus, the turbid solution becomes clear again. When the resultant clear solution is heated, calcium bisulfite decomposes to give back insoluble calcium sulfite along with SO2 and water. So, the clear solution becomes turbid again.
Ca(OH)2 + SO2→ CaSO3↓ + H2O
CaSO3 + SO2 + H2O→Ca(HSO3)2 (soluble)
KSEEB Class 11 Chemistry Calcium Compound Examples
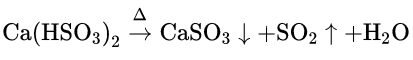
5. Reaction with acid:
Calcium hydroxide being a quite strong base reacts with acids and acidic oxides to form the corresponding salts and water.
Examples:
Ca(OH)2 + 2HCl→CaCl2 + 2H2O
Ca(OH)2 + H2SO4→ CaSO4 ↓+ 2H2O
Ca(OH)2 + CO2 →CaCO3↓- + H2O
Ca(OH)2 + SO2→ CaSO3 + H2O
3Ca(OH)2+ P2O5→ Ca3(PO4)2 + 3H2O
In its reaction with sulphuric acid, insoluble white calcium sulfate is formed and it forms a protective coating on solid Ca(OH)2 and stops the reaction.
Reaction with ammonium salts: Being a stronger base than ammonia, calcium hydroxide displaces ammonia from its salts when heated with an ammonium salt.
Examples: 2NH4Cl + Ca(OH)2→ 2NH3T↑ + CaCl2 + 2H2O
This reaction is generally used for the preparation of ammonia in the laboratory.
6. Reaction with chlorine:
At about 313K, chlorine gas reacts with slightly moist slaked lime to form a dry, white, and powdery substance with a pungent smell. This powder having bleaching and disinfecting properties is commonly called bleaching powder. Regarding the formation and . composition of bleaching powder, the following two views have been proposed.
1. According to Odling (1861), bleaching powder Is a mixture of calcium hypochlorite, Ca(OCl)2, and calcium chloride, CaCl2, and Is called calcium chlorohypochlorite, Ca(OCl). Its formation can be shown as follows
2Ca(OH)2+ 2Cl2 → Ca(OCl)2 + CaCl(Bleaching powder) +2 H2O
Or, 2Ca(OH)2 + 2CI2 → 2Ca(OCl)Cl + 2H2O
Or, Ca(OH)2 + Cl2 → Ca(OCl)Cl(Bleaching powder) + H2O
2. According to Bunn, Clork, and Ghifford (1935), bleaching powder is supposed to be a mixture of
Ca(OCl)2, CaCl2 and Ca(OH)2. Its formation can be represented as
2Cl2 + 3Ca(OH)2→ Ca(OCl)2-Ca(OH)2-CaCl2-2H2O (Bleaching powder)
Wlien Cl2 gas is passed through excess of cold lime water, calcium chloride, and calcium hypochlorite are formed.
2Ca(OH)2 + 2CI2→ CaCl2 + Ca(OCl)2 + 2H2O
When excess chlorine is passed through hot lime water calcium chloride and calcium chlorate are formed.
6Ca(OH)2 + 6Cl2→ 5CaCl2 + Ca(ClO3)2 + 6H2O
Uses of calcium hydroxide
CaCO3: Ca(OH)2 + CO2→ CaCO3 + H2O
3. Calcium carbonate (limestone), CaCO3
Natural occurrence: In nature, calcium carbonate occurs in large quantities as limestone, marble, calcite, chalk, etc. It occurs also in the mineral, dolomite which is the double carbonate of calcium and magnesium (CaCO3-MgCO3). Besides these minerals, CaCO3 occurs in abundance in corals, eggshells, outer covering of oysters, snail’s conch, and in teeth and bones of man and animals.
Calcium Compounds In KSEEB Chemistry Class 11
Preparation of calcium carbonate: Calcium carbonate may
Be prepared by passing CO2 through lime water or by adding a solution of Na2CO3 to a solution of CaCl.
1 Ca(0H)2 + CO2 → CaCO3↓+ H2O
CaCl2 + Na2 CO3→ CaCO3↓+ 2NaCl
The precipitate of CaCO3 is known as precipitated chalk.
Calcium carbonate Physical properties:
Calcium carbonate Chemical properties:
1. The action of heat:
When heated at a higher temperature ( ~ 1270K), calcium carbonate decomposes to give calcium oxide (quicklime) & carbon dioxide. The reaction is reversible and endothermic. So, it proceeds towards completion when the reaction is carried out in an open vessel.
CaCO3 ⇌ CaO + CO2 ↑-heat
2. Reaction with dilute acids: It reacts with dilute acids to form the corresponding calcium salts.
CaCO3 + 2HCl → CaCl2 + CO2↑ + H2O
CaCO3 + H2SO4 → CaSO4 + CO2↑ + H2O
CaCO3 + 2HNO3 → Ca(NO3)2 + CO2↑+ H2O
3. Reaction with carbon dioxide:
When CO2 gas is passed through a fine suspension of calcium carbonate in water, the latter slowly dissolves to produce calcium bicarbonate and thus, a clear solution is obtained.
CaCO3 + CO2 + H2O→ Ca(HCO3)2
When the resultant clear solution is heated, calcium bicarbonate decomposes back to insoluble CaCO3. Thus, the clear solution becomes turbid again. ‘
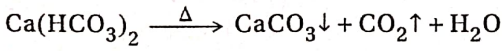
Uses of calcium carbonate:
4. Plaster of Paris (hemihydrate of calcium sulfate), (CaSO3)2-H2O
Preparation of Plaster of Paris:
Plaster of Paris is prepared by heating gypsum, (CaSO4-2H2O) at about 383-393K in a rotating burner.
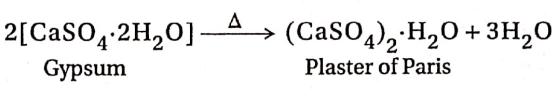
Properties of Plaster of Paris:
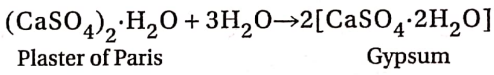
Key Compounds Of Calcium In KSEEB Syllabus Class 11 Chemistry
Uses of Plaster of Paris:
5. Cement
Cement is a mixture of finely powdered calcium silicates and aluminates along with small quantities of gypsum.
The raw materials used for cement are:
Cement Composition:
Different types of cement have different compositions. The composition of Portland cement is given below:
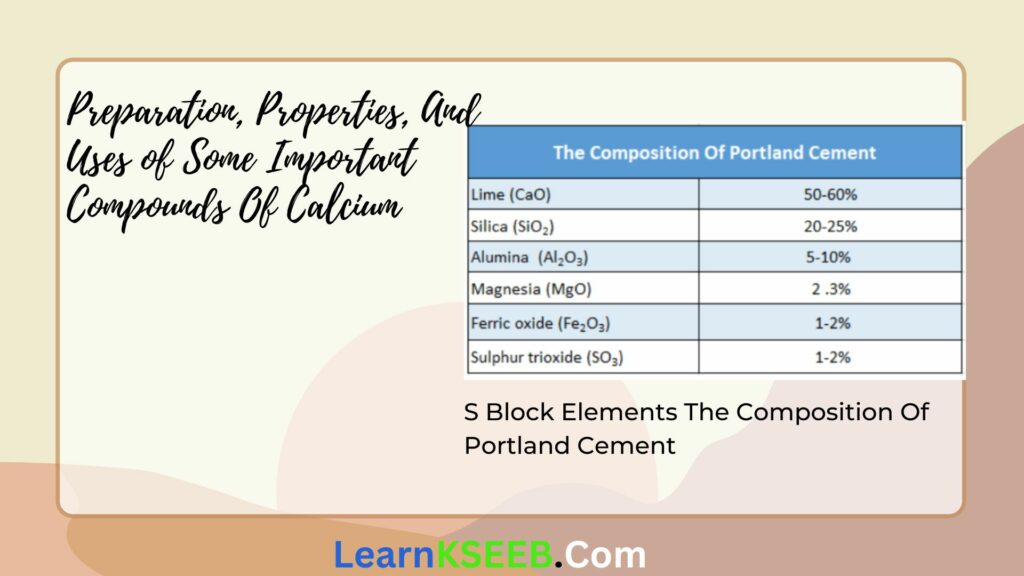
For cement to have good quality, the ratio of silica to alumina should be between 2.5 to 4 and the ratio of CaO to the total oxides of silicon, aluminum, and iron should be close to 2.
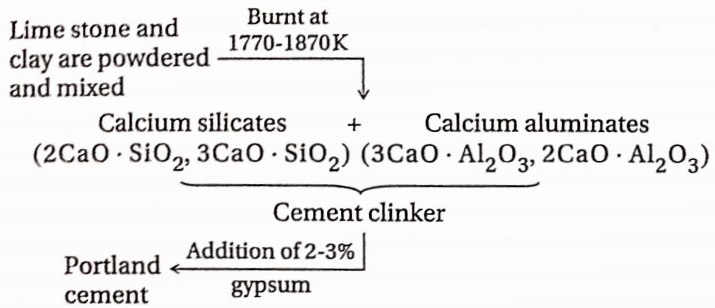
Cement Preparation:
For the manufacture of cement, limestone, and clay are fused by strong heating to form cement clinker. This is mixed with the gypsum and ground to a very thin powder.
Cement Settings:
When cement is mixed with water, it forms a plastic mass. After some time it becomes solid. This change is due to the three-dimensional linking between — Si—O — Si — and —Si—O—Al — chains. This transition from plastic to solid is called setting.
Fly ash is a waste product of the steel industry produced mainly due to the burning of coal and carbon compounds. It has similar properties to that of cement. Sometimes fly ash is used with the cement to reduce the cost without compromising on the quality.
KSEEB Class 11 Chemistry Calcium Compound Examples
Cement Uses:
In nature, water is an indispensable component. 97% of the In the nature, water is an indispensable component—97% of the water, which is practically of no use to human beings.

Read and Learn More KSEEB Class 11 Chemistry Notes
Water pollution:
When the water of different water bodies gets contaminated with one or more chemical substances, evolved either by natural phenomena or indiscriminate human activities and tend to cause health hazards to man and other living beings or adversely affect the processes of their livelihood, then the water is said to be polluted.
1. Domestic wastes
Solid waste of various materials of domestic use
For example: Discarded paper, plastics, torn cloth, vegetable refuse, remains of food etc.),
Excreta of man and domestic animals are mostly left in open places. With time, these discarded materials are carried by wind or rainwater to the nearby water bodies. This contaminates the water and makes it unfit for use.
KSEEB Class 11 Chemistry Notes On Water Pollution
Water pollution caused by domestic wastes:
1. Domestic wastes mostly contain organic compounds. These organic compounds are decomposed by bacteria in the presence of dissolved oxygen (DO). This process is called biodegradation.
In the process of biodegradation, carbon, hydrogen, nitrogen, phosphorus etc., present in the organic compounds are oxidised to CO2, H2O, nitrate, phosphate and other salts. During the process the quantity of dissolved oxygen gradually decreases. Naturally, aquatic plants, fi hes and other aquatic organisms do not get sufficient oxygen for respiration.
Consequently, aquatic living beings face serious problems. DO is considered to be an important parameter in predicting the quality of water. For aquatic plants and animals, the value of DO must not be less than of 4-6mg L-1 . With the increasing value of DO, the quality of the water gradually improves. Lowering the value of DO indicates that the water is getting polluted.
Biochemical Oxygen Demand or BOD:
Biochemical oxygen demand (BOD) may be defined as the number of milligrams of oxygen required for biodegradation (i.e., biochemical degradation) of organic matter present per litre of polluted water.
Chemical Oxygen Demand or COD :
2. If dissolved oxygen is deficient in water the oxidation of organic pollutants does not get completed. As a consequence of incomplete oxidation, methane (CH4), hydrogen sulphide (H2S), phosphine (PH3), different amino compounds etc., are formed which creates an extremely off ensive odour.
3. Waste materials, sewage from dispensaries, hospitals and domestic wastes carrying pathogenic microorganisms are drained into the water bodies which may result in various diseases such as cholera, typhoid, paratyphoid, dysentery, hepatitis, polio, gastroenteritis, jaundice etc.
4. Waste materials like plastics do not undergo bacterial decomposition in the presence of oxygen, i.e., they are nonbiodegradable. They remain unaffected even if they areleft in water for years. Thus, they decrease the depth of water as well as increase the extent of water pollution under the influence of their constituent chemical compounds
Water Pollution Notes For KSEEB Class 11 Chemistry
2. Industrial wastes
1. Cadmium (cd):
Refining of zinc, copper and lead, electro¬ plating industries, iron and steel factories, Ni-Cd battery factories etc., release cadmium as waste material into rivers and other water reservoirs.
Harmful effects:
Cadmium, introduced into the body through water pollution causes vomiting, irritation of the lungs, malfunctioning of the liver and kidney high blood pressure, anaemia, disorder of bone marrow etc.
Ital-llai disease:
2. Mercury (Hg):
The water discharged as industrial waste from the factories producing caustic soda, chlorine, pesticides etc., is die source of mercury pollution.
Harmful effects:
Mercury is highly toxic. It causes stomach pain, dropsy (oedema), headache etc. Moreover affects the nervous system and kidneys, decreases the reproductive power of males, and babies are found to be born with deformity.
Minamata disease:
In 1953-69, die disease caused by pollution due to mercury appeared in the Minamata area on the sea-coast of Japan and came to be known as Minamata disease. In that area, more than 100 people died of this disease and thousands of people became crippled. In this coastal region, waste materials contaminated with mercury from a polyvinyl factory were regularly discharged into sea¬ water.
Mercury present in the effluent was converted into highly poisonous methyl mercury by different reactions. This poisonous compound was introduced into human bodies through sea fishes and led to the outbreak of Minamata disease. The primary symptoms of this disease are the lack of sensation in muscles, lips, tongue etc., which culminate in blindness and loss of memory
3. Lead (Pb):
The waste materials discharged from factories For example: extraction and refining of lead, paints, varnishes, alloys, batteries and ship-building etc.), containing lead, pollute the water of rivers, lakes and other sources. Apart from these, the anti-knocking compound [Pb(C2H5)4] used in gasoline and petrol is a potential source of lead
Harmful effects:
If water contaminated with lead enters the body, lead gets accumulates in the body. Most of the lead is ultimately deposited in the bone. Lead poisoning gives rise to symptoms such as loss of appetite, vomiting, constipation, anaemia, insomnia, headache etc., and also affects the digestive system.
4. Manganese (Mn):
Effluents containing Mn from ferromanganese producing industries, welding factories and MnO2 as waste materials released from dry batteries, mix with water as pollutants.
KSEEB Chemistry Class 11 Water Pollution
Harmful effects:
If Mn-containing water is consumed for a prolonged time, it causes nervous disorder.
5. Coball (Co):
Industrial discharge from ceramic, paint or dye industries results in the Co-pollution of water.
Harmful effects: If cobalt-contaminated water is consumed, symptoms such as lowering of blood pressure, diarrhoea, deformation of bones etc., are developed.
6. Arsenic (As):
The main sources of arsenic poisoning are pesticides, chemical wastes, pharmaceutical industries, mining by-products etc. In the tube well water in some places, arsenic compounds are present.
Harmful effects:
Water polluted by arsenic disturbs blood circulation in the skin and black or ash spots appear on the skin of the throat, neck and back. The skin of the hands and legs becomes rough and spots like moles appear on the skin. Continuous use of water containing arsenic for a longer time causes cirrhosis of the liver, cancer in the lungs and urinary track
7. Arsenic pollution:
According to the recommendations of the World Health Organisation (WHO), water containing 0.01 mg of arsenic per litre is quite safe for drinking. The limit of arsenic in water that human bodies can sustain is 0.05 mg L-1. But the average arsenic content in the tube well water of some places in the vast region of Bangladesh including some districts of Gangetic West Bengal
For example:
North and South 24-Parganas, Nadia, Murshidabad, Maldah etc.) is 0.25mg. L-1 . As a result of the indiscriminate use of this water, nearly ten lakhs of people have been victimised in West Bengal. Out of these, at least two lakhs of people have been suffering from acute skin diseases
8. Black-foot disease:
Consumption of arsenic contaminated for a long period also causes severe damage to. lower limbs and formation of black lumps on palm and foots. This is known as ‘black-foot disease.
3. Fertilisers used in agriculture
Chemical fertilisers or nutrients are extensively used for increasing the agricultural production. Mainly urea or organic fertilizers and ammonium sulphate, ammonium nitrate, monocalcium phosphate etc., are used as inorganic fertilisers. A certain portion of these fertilisers remains unutilised and being carried by the rainwater, falls into the nearby, lakes etc., and thus causes water pollution
Water containing nitrate ions cannot be used as potable water because nitrate ion cannot be removed by the usual process of purification of water. Consumption of such water affects the haemoglobin of babies, causing the disease called ‘blue baby syndrome’. Moreover, nitrate ions are converted to carcinogenic nitrosamines inside the body
Eutrophication:
The ill effects of this over-nutrition i.e., eutrophication may be summarised as:
Key Points On Water Pollution In KSEEB Class 11 Chemistry
4. Pesticides used in agriculture
A wide range of synthetic organic chemicals are used for the better production and preservation of crops. For example, insecticides, fungicides, herbicides etc., are applied to the field to kill insects, fungi, herbs etc. These chemicals are collectively known as pesticides. Pesticides, when used in agricultural fields, are carried by flowing water. Thus, they enter the hydrosphere and cause pollution of water. Again, when pesticides are sprayed in the field, a part of them get mixed with the atmosphere which come down along with rain water and mixes with the water of the rivers, lakes etc. Water pollution is also caused by the wastes discharged from the factories producing pesticides.
Different classes of pesticides and their harmful effects:

Biomagnification:
There are some pollutants which do no There are some pollutants which do no aldrin, heptachlor etc. These compounds exist for years together, keeping their poisonous effect Intact. These are called permanent organic pollutants.
They are not soluble in water but soluble in fats and oils. So they dissolve in body fats and go on accumulating. These highly toxic substances accumulated in living bodies are transmitted to the bodies of other living beings through food chains. These persistent organic pollutants (POP) exist at highly toxic levels in the bodies of living beings owing to repeated consumption of polluted food
5. Detergent
Detergent is widely used as a cleaning agent in household work and in industry. The effluent released after its use mixes with the nearby ponds, rivers etc., and causes water pollution. Two chief constituents of detergent are—
Water pollution caused by surface active agents:
1. Surface active agents decrease the surface tension of water and consequently help in the formation of foam emulsion and oily substances with water. These surface active agents are non-biodegradable and thereby entail water pollution.
2. Foam created by detergents forms a layer on the surface of water and thus prevents water from coming in contact with air and sun rays. Consequently, water cannot absorb oxygen from air and the dissolved oxygen level (DO) in water decreases.
3. Furthermore, sun rays being obstructed, the aquatic plants at the bottom cannot release oxygen by the process of photosynthesis. For this reason, also, the dissolved oxygen level gradually gets diminished. This results in the deficiency of oxygen required for the respiration of aquatic plants and animals.
4.. Surface active agents form a layer on some organic pollutants
For example Phenolic compounds
So, phenolic compounds present in water can no longer come in contact with bacteria and hence the biodegradation of organic pollutants becomes inhibited. Consequently, the extent of pollution in water increases.
Pollution caused by builders or fillers
Detergent contains phosphate salts known as builders or fillers. Phosphate ions produced from them form water-soluble complexes by combination with the basic radicals Ca+2, Mg+2 etc. Iff These complex phosphate salts serve as nutrients for algae and aquatic plants, consequently affecting their rapid population growth (Eutrophication). Plenty of oxygen is required for their respiration. This results in rapid decrease in the level of dissolved oxygen (DO) and the survival of aquatic animals becomes extremely difficult.
6. Radioactive substances
Radioactive substances, during mining and refining as well as from nuclear power plants, may be carried into water. Radioactive discharges from medical and scientific institutions using radioactive isotopes may also lead to water pollution.
Harmful effects:
The presence of radioactive substances in trace amounts may cause nervous debility, physical deformity, miscarriage, sterility, cancer, blindness etc. The harmful influence of this radioactivity continues from one generation to another.
7. Thermal pollution
In hydroelectric power plants, generally, the water from rivers or lakes is converted into superheated steam which is used to rotate the turbine. Only a negligible fraction of heat carried by steam is transformed into electrical energy and the rest returns to rivers or lakes with the help of water. This process continues, in cyclic order.
As a result, the temperature of water of the river or the lake rises considerably and the dissolved oxygen (DO) level decreases, causing great harm to the aquatic animals, particularly the fishes. In thermal nuclear power plants and many other industries, water is used as a coolant, which is discharged at a high temperature to rivers or lakes resulting in a rise in the temperature of the water. This increased temperature accelerates the faster assimilation of the waste materials, causing the depletion of dissolved oxygen (DO).
KSEEB Class 11 Chemistry Water Pollution Solutions
8. Oil-slicks on sea-water
Mineral oils and by-products of oil spread into seawater for several reasons. Consequently, a layer of floating oil (oil slicks) on sea-water is formed and the transfer of atmospheric biochemical level oxygen dissolved of into dissolved oxygen decomposes sea-water(DO)oxygen levels is prevented reduced
9. Controls of water pollution
Soil is a constantly changing mixture of materials composed of organic and inorganic substances water air microorganisms etc., which allows plants to grow. Various kinds of organic and inorganic materials, mixed with ground are extremely difficult. rocks, give rise to the formation of soil.
Chemical Composition Of Soil:
Soil is a complex substance. Its various constituents are:
1. Minerals:
Soil contains different kinds of minerals. The chemical nature of the rock from which the soil originates determines the variety and quantity of minerals in it. The particles present in the soil are basically silicate minerals. The chief constituent elements of soil are silicon, calcium, sodium, potassium, magnesium, iron, aluminium, oxygen etc. These elements are present in the form of silica (SiO2), silicate (KAISi3O8, NaAlSi3O8), epidote [4CaO, 3(AlFe)2O3, 6SiO2, HO2] etc.

Read and Learn More KSEEB Class 11 Chemistry Notes
2. Air:
The particles of soil leave enough space in between, which is occupied by air. The air present in the soil contains carbon dioxide, oxygen, nitrogen and water vapour. But the quantity of O2 present in the soil is less than that of O2 present in air while the quantity of CO2 in the soil is comparatively greater than the corresponding amount in air.
3. Water:
Water content in the soil always varies from place to place. The constitution of soil determines its water tension capacity. If the amount of organic compounds present in the soil is increased then the water-retention capacity of soil will also increase. This water serves as a solvent for mineral and organic matter. Moreover, water retained in the soil plays a vital role in maintaining the structural arrangement of the soil.
4. Organic compounds:
Generally, organic substances are produced from the remnants of dead plant and animal bodies. Besides this, the waste material of living beings is also a potential source of organic substances. The organic matter liberated due to the bacterial decomposition of the remains of plant and animal bodies mix with soil to form humus. This humus is a very significant part of the soil.
KSEEB Class 11 Chemistry Notes On Soil Pollution
Some of its qualitative features are:
Effect of soil on the environment:
For example:
Sodium chloride from the soil. The animal kingdom survives by taking this organic food, otherwise, the existence of animal life would have been endangered. So, soil is a component of immense importance for the living world i.e., the flora and fauna
Soil pollution is caused when industrial wastes, radioactive pollutants, domestic and municipal wastes, and agricultural pollutants, are either thrown or dumped into the soil. These reduce the overall quality of soil and are harmful for living beings.
1. Various sources of soil pollution:
1. Pollution caused by industrial waste:
Plastic and paint industries, coal and mining industries, metallurgical units and the industries for the production of sugar, leather, cotton, pesticides, glass, cement etc., discharge a large amount of their discarded waste, which causes soil pollution.
2. Pollution caused by municipal waste disposal:
Waste material discharged by municipalities
KSEEB Chemistry Class 11 Soil Pollution
3. Pollution caused by fertiliser:
4. Pollutionbypesticides:
The harmful effects of these pesticides are as follows:
5. Pollution caused by acid rain:
Pollution caused by radioactive substances:
Radio¬ active waste materials, emitted from atomic reactors, as a result of experimental studies on atom bombs and nuclear experiments, are added to the soil. The radioactive emissions from the waste pollute the whole environment including the land mass.
2. Controls of soil pollution
Chemistry is undeniably an important part of our lives since it leads to the formulation and fabrication of medicines, materials, polymers, paints, coatings, electronics etc. Chemists also address fundamental problems like global warming, ozone layer depletion, soil and water pollution, efficient food production via photosynthesis etc.
However, processes on an industrial scale not only produce the desired material, but also large quantities of undesired and toxic chemicals in the form of solids, liquids and gases and have become the biggest challenge that chemists need to face. Hence, there has been a considerable effort to shift to synthetic methods which would minimise environmental pollution. This is where the concept of green chemistry steps in
Read and Learn More KSEEB Class 11 Chemistry Notes
The U.S. Environmental Protection Agency (USEPA) defines green chemistry as the design of chemical products and processes that reduce the generation of hazardous substances. The use and production of these chemicals and processes may involve reduced waste products, non-toxic components and improved efficiency. Green chemistry is a highly effective approach to pollution prevention because it applies innovative scientific solutions to real-world environmental situations. Green chemistry is also known as sustainable chemistry.
A key difference between environmental chemistry and green chemistry: Environmental chemistry deals with the study of chemical pollutants in the environment whereas green chemistry is concerned with the design of chemicals and processes that minimise toxicity to the environment. This is the key difference between environmental chemistry and green chemistry

1. Applications of Green Chemistry
The term green chemistry was coined by P. T. Anastas who elucidated the principles of green chemistry in his book ‘Green Chemistry:
2. Contribution of green chemistry
A few classic chemical processes where green chemistry has proved beneficial are outlined below.
KSEEB Class 11 Chemistry Notes
1. Synthesis of Ibuprofen:
Ibuprofen is the active ingredient of several analgesic and anti-inflammatory drugs. The initial synthesis of ibuprofen consisted of a six-step process with a very poor atom economy. However, recent advances has made possible the synthesis of ibuprofen with an atom economy of more than 90%. This synthesis produces less waste and is a three-step process.
2. Use of dense-phase carbon dioxide:
Dense-phase carbon dioxide is used in both homogeneous and heterogeneous catalysis. Its use allows us to replace organic solvents with chemically inert and environmentally non-toxic carbon dioxide. It is used in the food industry as a reusable solvent to ensure minimal nutrient loss and better preservation of the food products. Dense-phase carbon dioxide may also be used to enhance the quality of cement and to reduce the industrial waste of coal plants.
3. Use of liquid carbon dioxide in dry cleaning:
Carbon dioxide is a new environment-friendly alternative for dry’ cleaning. Liquid carbon dioxide effectively removes stains. At the same time, it is less harmful than perchloroethylene, the solvent which is used by 80% dry cleaners.
4. Use of carbon dioxide as a refrigerant:
Chlorofluorocarbons (CFCs) have been extensively used as refrigerants. However, CFCs are now known to be the prince reason for the ‘ozone hole’ in the stratosphere. Carbon dioxide is now used as a refrigerant and has zero ODP (ozone depletion potential) and minimal CWP (global warming potential).
5. Catalytic hydrogenation of diethyl amine:
A greener approach to the catalytic hydrogenation of diethyl amine furnishes a herbicide with the least environmental toxicity.
6. Antifouling agent Sea-Nine:
Sea-Nine (The Dow Chemical Company) is a rapidly biodegradable settlement inhibitor. It is a highly effective antifoulant against bacterial slime, algae, hydrozoids, etc., and is free from heavy metals. Sea-Nine is a good alternative to organotin compounds which cause aquatic toxicity
7. Paper industry and laundry:
Chlorine has long been used for producing good quality paper from wood (by removing all lignin). However, the use of chlorine leads to the formation of chlorinated hydrocarbons which are known to be potential. H2O2 is now being used as an alternative in the presence of some activators. The use of H2O2 produces lesser environmental concerns.lt is also used in laundry leads lesser use of water.
Green Chemistry notes for Class 11 Chemistry KSEEB
8. Pyrocool foam:
Pyrocool is used in portable fire extinguishers. It uses a non-toxic foam that cools and extinguishes fire without causing risk to human life. It is also free from volatile organic compounds (VOCs), CFCs and carcinogenic chemicals.
9. Synthesis of antibiotics:
Antibiotic drugs like ampicillin and amoxicillin can be synthesised by biochemical methods using environment-friendly enzymes.
10. Sonochemistry:
Sonochemistry deals with the study of chemical reactions induced by sound waves.
11. Single-step synthesis of ethanal:
A single-step synthesis of ethanal from ethyl alcohol has been studied. This method employs water-soluble ionic catalysts and is environmentally friendly.
12. Fuel cell:
A new variety of fuel cells have been fabricated which can be used as batteries in cell phones. Such fuel cells are based on the combustibility of ethanol.

Chapter Wise KSEEB Class 8 Social Science Notes Pdf free download was designed by expert teachers from latest edition of KSEEB books to get good marks in board exams. KSEEB Class 8 Social Science Notes contains History, Geography and Civics notes of all chapters are part of Revision Notes for grade 8 Social Science. Here we have given notes Class VIII Social Science.
Part A – Our PASTS – III (History)
Part B – Resources and Development (Geography)
Part C: Social and Political Life -III (Civics)