KSEEB Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure Important Concepts
Elements, compounds and mixtures. Homogeneous and heterogeneous mixtures, colloids and suspensions.
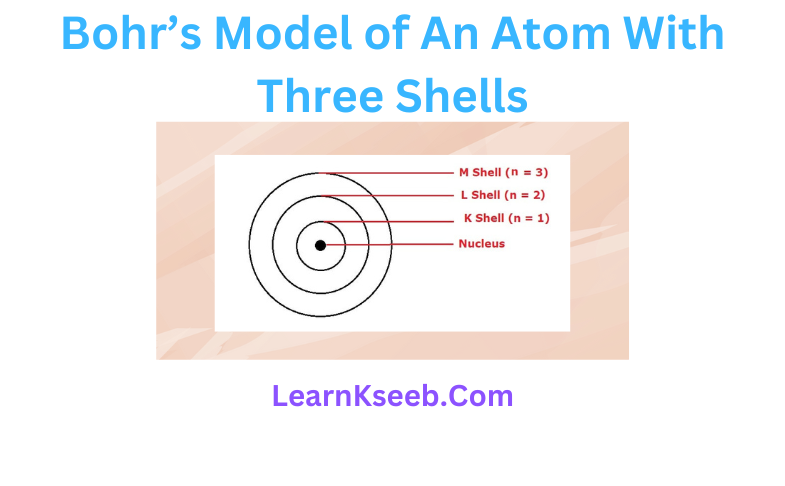
Matter or Substance
Matter around us is of two types:
1. Pure substances
2. Mixtures
Pure substance
A material which contains only one kind of atoms or molecule.
Eg: Hydrogen, oxygen, chlorine etc.
Pure substances are uniform throughout because it consists of only one kind of particle.
Eg: Water, sodium chloride etc

Read and Learn More KSEEB Solutions for Class 9 Science
Mixture
A mixture contains more than one element or compound in any ratio.
Eg: Salt solution
Types of mixtures
Mixtures are of two types:
1. Homogeneous mixture
2. Heterogeneous mixture
Homogeneous mixture has uniform composition.
Eg: Sugar in water, water in alcohol.
Heterogeneous mixture does not have uniform composition.
Eg: Oil and water, salt and sulphur
| Class 9 Social Science | Class 9 Science | Class 9 Maths |
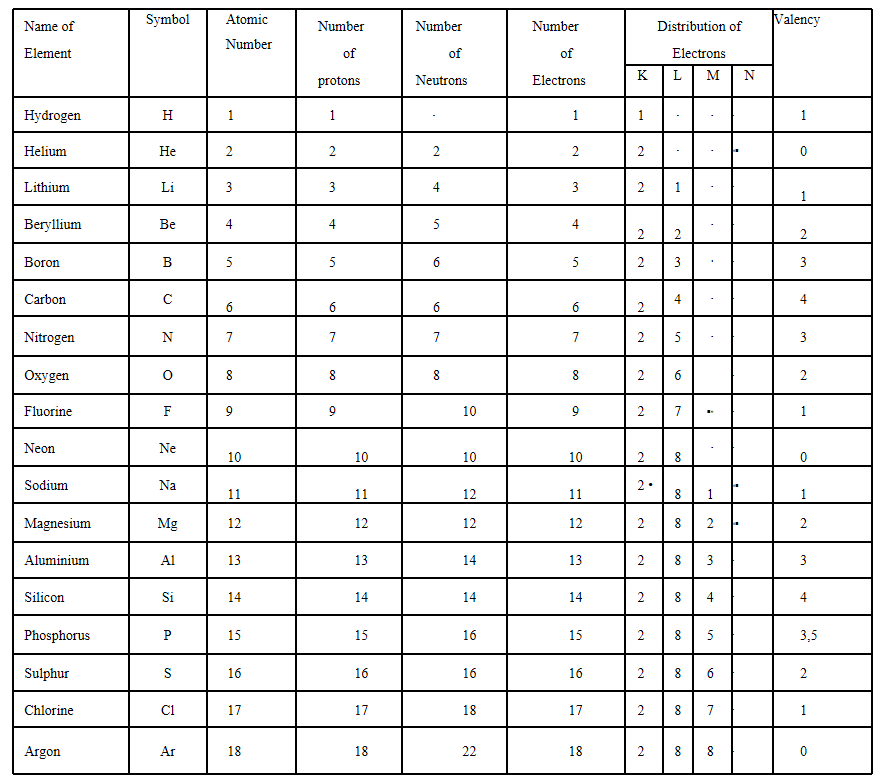
Elements
An element is defined as the simplest form of the basic form of a pure substance which can neither be broken into nor built up from simpler b. Colloidal solution
substances by any physical or chemical method.
Compounds
A pure substance made up of two or more elements chemically combined together in a fixed
proportion by mass.
Solution
Solutionis a homogenous mixture of two or more substances.
Solute
The component of solution which is present in small amounts is called solute. Eg: Salt is a solute in salt solution.
Solvent
The substance which is present in large amounts is called solvent. Eg: Water is a solvent in salt solution.
Aqueous solution
The solutions that are made in water.
Is Matter Around Us Pure KSEEB Class 9 Question Answers
Concentrated solution
The concentration of solution can be specified by measuring amount of solute dissolved per litre of solution.
Saturated solution
A solution in which no more solute can be dissolved at a particular temperature.
Unsaturated solution
A solution which contains less amount of solute than required to make it saturated.
Supersaturated solution
It is a solution which contains more dissolved solute than the saturation concentration.
Types of solution
Based on the size of solute particles solution can be classified into:
1. True solution
2. Colloidal solution
3. Suspension
Dispersion medium
The medium in which colloidal particles are dispersed is called the dispersion medium.
Eg: Water is the dispersion medium in milk.
Tyndall effect
When a beam of light is passed through a colloidal solution placed in a dark place, its path becomes clearly visible. It is due to scattering of light by the colloidal particles.
Eg: When a beam of light is thrown on screen observed in theatres.
Brownian movement
Constant random motion of colloidal particles in a zigzag path is called Brownian movement.
Eg: Abeam of fight enters a room through a small hole, dust particles move in a zigzag manner.
Centrifuging and its applications
Centrifuging is a process in which denser particles are forced to settle at the bottom and the lighter stay at the top when rotated at high speed.
Applications:
1. It is used in Diagnostic Laboratories for blood and urine test.
2. Used in washing machines to squeeze out water from wet clothes.
3. Used in dailies to separate butter from cream.
Crystallization and its applications
Crystallization is a process in which hot saturated solution of a compound in suitable solvent is cooled so as to get crystals of a pure substance.
Applications:
1. Purification of salt that we get from sea water.
2. Separation of crystals of alum from impure samples like Potash alum, copper sulphate etc.
Chromatography and its application
Chromatography is a process of separation of those solutes which dissolve in the same solvent.
Applications:
1. It is used to separate colours of the dye.
2. To separate pigments from natural colours.
3. To separate drugs from blood.
Applications of separating funnel
1. It is used to separate immiscible liquids having differences in their densities.
2. In the extraction of iron from its ore.
Miscible liquids
The liquids which mix with each other are called miscible liquids.
Immiscible liquids=
The liquids which do not mix with each other are called immiscible liquids.
Physical change
Those changes in which only the physical properties of a substance change but no new substances are formed.
Eg: Breaking of glass tumbler, melting of wax.
Chemical change
Those changes in which new substances are formed.
Eg: Burning of magnesium ribbon, rusting of iron.
Metals, non-metals and metalloids
Metals: They are malleable and ductile, good conductors of heat and electricity.
Eg: Iron, copper, silver etc.
Non-metals: They are neither malleable nor ductile, non-conductors of heat and electricity.
Eg: Hydrogen, oxygen sulphur etc.
Metalloids: They have properties in between those of metals and non-metals.
Eg: Silicon, germanium, arsenic etc.
Is Matter Around Us Pure Exercises
Question 1. Which separation techniques will you apply for the separation of the following?
1. Sodium chloride from its solution in water.
Answer Evaporation or distillation
2. Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
ammonium chloride.
Answer Sublimation
3. Small pieces of metal in the engine oil of a car.
Answer Filtration
4. Different pigments from an extract of flower petals.
Answer Chromatography
Class 9 Science Chapter 2 KSEEB Textbook Solutions
4. Butter from curd.
Answer Centrifugation
5. Oil from water.
Answer Separating funnel
6. Tea leaves from tea.
Answer Filtration
7. Iron pins from sand.
Answer Magnetic separation
8. Wheat grains from husk
Answer Sieving or winnowing
9. Fine mud particles suspended in water
Answer Adding alum
Question 2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer Take some water in a kettle and boil it. To the hot water add some tea leaves and boil. Add some sugar and milk and boil again. Filter the mixture through a sieve.
Solution – Tea filtrate
Solvent – Water
Solute – Sugar and milk
Dissolve – Sugar
Soluble – Caffeine in tea powder
Insoluble – Tea leaves
Filtrate-Tea
Question 3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below. (Results are given in the following table, as grams of substances dissolved in 100 grams of water to form a saturated solution)
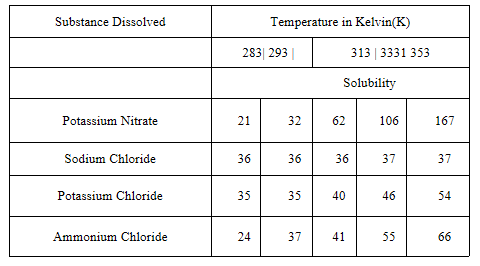
1. What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313K?
Answer Mass of Potassium Nitrate in lOOg of water at 313K = 62 g.
Mass of Potassium Nitrate in 50 g of water in the saturated solution at 313Kis
622
313K x50g =31g
lOOgg
2. Pragya makes a saturated solution of
potassium chloride in water at 353K and leave the solution to cool at room temperature. What would she observe as the solution cools? Explain.
Answer When a saturated solution of Potassium Chloride at 353K is cooled, the solubility of Potassium Chloride in water decreases. Asa result the amount of potassium chloride which exceeds its solubility at lower temperatures separates out as crystals.
3. Find the solubility of each salt at 293K. Which salt has the highest solubility at this temperature?
Answer
Solubility of PotassiumNitrate = 32g
Sodium Chloride = 36g
Potassium Chloride = 35g
Ammonium Chloride -37g
Therefore Ammonium Chloride has highest solubility at the temperature.
KSEEB Solutions for Is Matter Around Us Pure Short Notes
4. What is the effect of change of temperature on the solubility of a salt?
Answer The solubility of salt increases with temperature.
Question 4. Explain the following giving examples.
1. Saturated solution
Answer A solution in which no more solute can be dissolved at a particular temperature.
Eg: The stage where no more sugar will dissolve in water.
2. Pure substance
Answer A pure substance contains the particles of only one kind of matter.
Eg: Copper, hydrogen, oxygen.
3. Colloid
Answer They are heterogeneous mixtures of solute and solvent. In which particle size lies in between those of true solution and suspension.
Eg: Face cream, muddy water etc.
4. Suspension
Answer In a heterogeneous mixture the solute particles are visible to the naked eye.
Eg: Bleaching powder in water.
Question 5. Classify each of the following as a homogeneous or heterogeneous mixture. Wood, soda water, vinegar, soil, air, filtered tea
Answer
Homogeneous mixture: Sodawater, vinegar, filtered tea
Heterogeneous mixture: Wood, air & soil.
Question 6. How would you confirm that a colourless liquid given to you is pure water?
Answer If the boiling point and freezing point of the given liquid come out to be 100°C or 0°C respectively under 1 atmospheric pressure, we can confirm that the liquid is pure water.
Question 7. Which of the following materials fall in the category of a pure substance? Ice, milk, iron, hydrochloric acid, calcium oxide, mercury, brick, wood and air.
Answer Ice, iron, calcium oxide, hydrochloric acid and mercury are pure substances because they contain particles of only one kind of matter.
Question 8. Identify the solutions among the following mixtures. Soil, seawater, air, coal, soda water.
Answer Sea water and soda water are solutions.
Question 9. Which of the following will show “Tyndall Effect”? Soil solution, milk, copper sulphate solution, starch solution.
Answer Milk and starch show Tyndall Effect because the particles are big enough to scatter light.
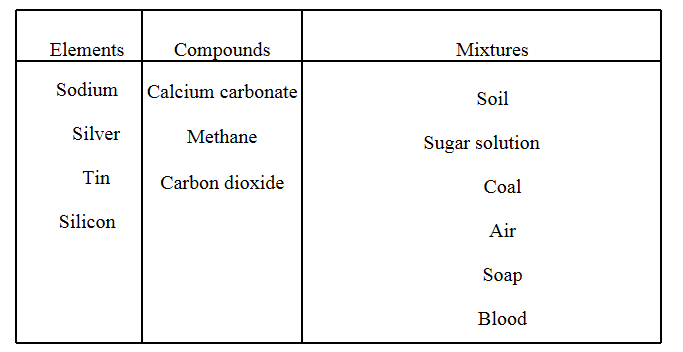
Question 10. Classify the following into elements, compounds and mixtures. Sodium, soil, sugar solution, silver, calcium carbonate, tin, silicon, coal, air, soap, methane, carbon dioxide and blood.
Answer
Question 11. Which of the following are chemical changes?
1. Growth of a plant
Answer Chemical change
2. Rusting of iron
Answer Chemical change
3 . Mixing of iron filings and sand
Answer Physical change
4. Cooking of food
Answer Chemical change
5. Digestion of food
Answer Chemical change
6. Freezing of water
Answer Physical change
KSEEB Class 9 Science Chapter 2 Important Questions
7. Burning of candle
Answer Chemical change
Is Matter Around Us Pure Textual Questions
Question 1. List the points of differences between homogeneous and heterogeneous mixtures.
Answer
Homogeneous mixture
It has uniform composition and properties throughout.
Eg: Sugar in water and alcohol.
Heterogeneous mixture
It has non-uniform composition and different parts having different properties.
Eg: Sand and salt, water in oil
Question 2. What is meant by a pure substance?
Answer A pure substance is a homogeneous material consisting of a single type of particle and definite set of properties.
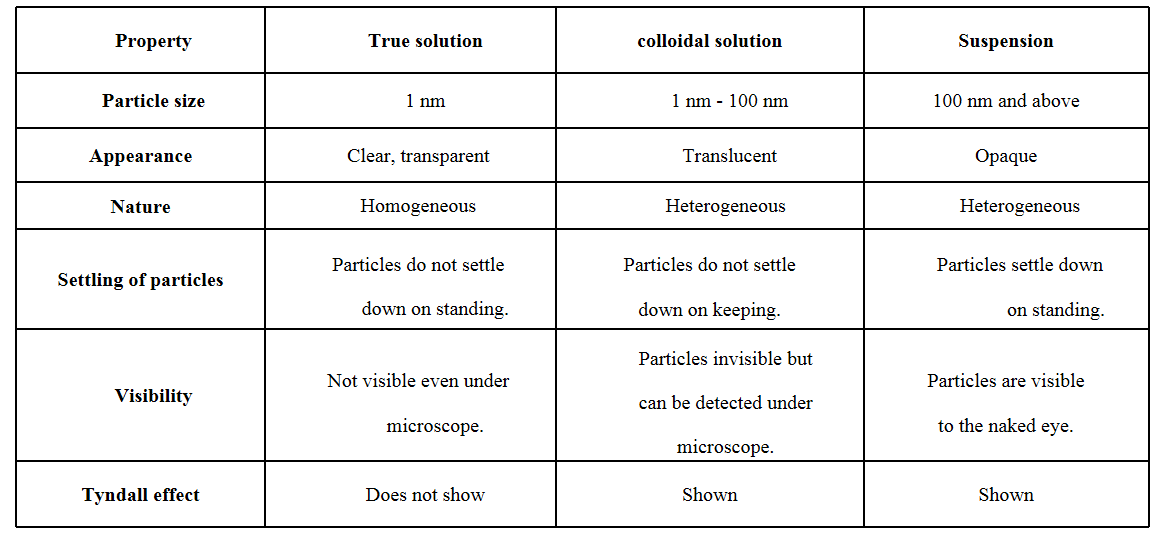
Question 3. How are true solution, colloidal solution and suspension different from each other?
Answer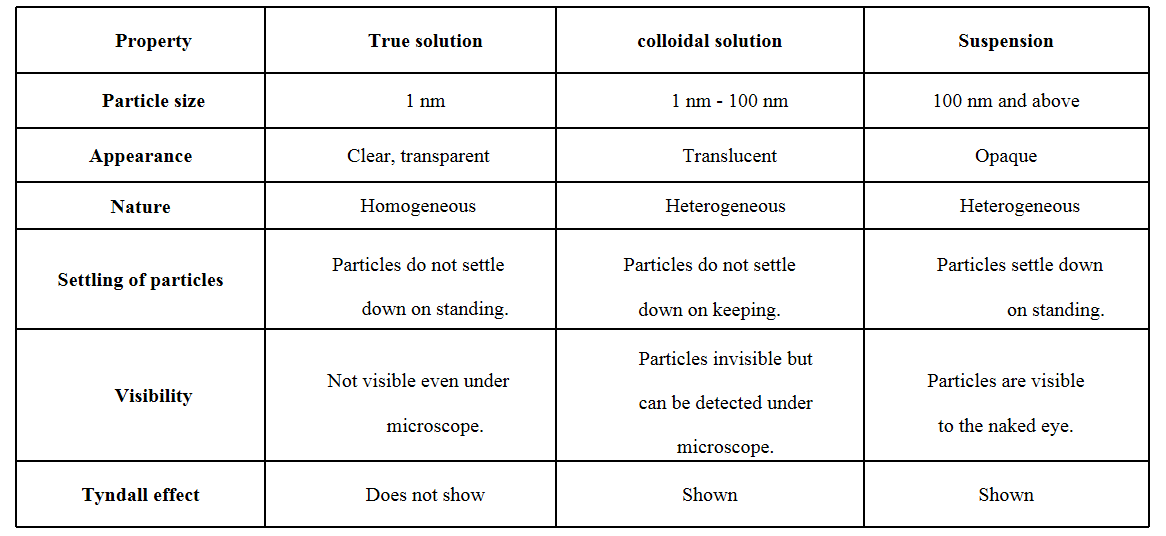
Question 4. To make a saturated solution, 36g of Sodium Chloride is dissolved in lOOg of water at 293K. Find its concentration at this temperature.
Answer Mass of sodium chloride = 3 6 g
Mass of water = lOOg Mass of sodium chloride + water = 100 + 36= 136g
Concentration of sodium chloride solution
Mass of solute
Mass of solution = 36 1000
136
=26.47%
Question 5. How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C) which are miscible with each other?
Answer The technique of simple distillation or fractional distillation is used.
Question 6. Name the technique to separate:
1. Butter from curd
Answer Centrifugation
2. Salt from seawater
Answer Crystallization or evaporation
3. Camphor from Salt
Answer Sublimation
Question 7. What type of mixtures are separated by the technique of crystallization?
Answer Homogeneous mixtures of a pure solid in the form of its crystals from solution are separated by the technique of crystallization.
Question 8. Classify the following as chemical or physical changes.
1. Cutting of trees
Answer Physical change
2. Melting of butter in a pan
Answer Physical change
3. Rusting of almirah
Answer Chemical change
4. Boiling of water to form steam
Answer Physical change
5. Passing of electric current through water and the water breaking down into hydrogen and oxygen gases.
Answer Chemical change
KSEEB Solutions Chapter 2 pure substances And Mixtures Class 9
6. Dissolving common salt in water
Answer Physical change
7. Burning of paper and wood
Answer Chemical change
Question 9. Try segregating the things around you as pure substances and mixtures.
Answer Pure substances: Water, sodium chloride, dry ice, sugar, pure metals.
Mixtures: Milk, soil, minerals, alloys, cool drinks.
Is Matter Around Us Pure Additional Questions
Question 1. Classify the following as sol, solution and suspension.
1. Milk of Magnesia
Answer Sol
2. Coloured gemstones
Answer Solid sol
3. Aerated drinks
Answer Solution
4. Emulsion paint
Answer Emulsion
Question 2. What is tincture in Iodine? Identify solute and solvent in tincture of Iodine.
Answer A solution of iodine in alcohol is known as tincture of Iodine.
Solute – Iodine
Solvent-Alcohol
Question 3. Can we separate alcohol dissolved in water by using a separate funnel?
Answer Both alcohol and water are miscible and hence cannot be separated by using a separating funnel.
Question 4. Give an example of aqueous and nonaqueous solution.
Answer Aqueous – Acetone water
Non-aqueous – Ether-Benzene
Question 5. A solution contains 60 g of common salt in 240 g of water. Calculate the concentration in terms of mass by mass percentage of solution.
Answer Mass of solution = Mass of solute + Mass of water.
= 60 + 2401| 300 g 60
Mass by mass percentage = x 100 = 20
Question 6. Smoke and fog both are aerosols. In what way are they different?
answer the dispersion medium of both smoke and fog is air and hence they are called aerosols. They are different from each other in the dispersed phase. In smoke dispersed phase consists of fine particles of gas. While in case of fog it consists of particles of water.
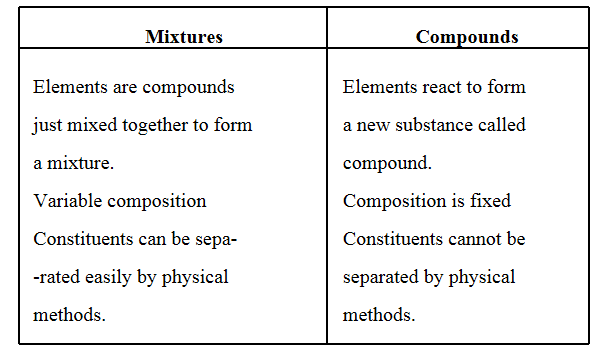
Question 7. Differentiate between mixtures and compounds.
Answer
Question 8. Name the solute and the solvent for the following mixtures.
1. Tincture of Iodine
2. Soda water
3. Sugar solution
Answer.
1. Tincture of Iodine – Solute is iodine and solvent is alcohol.
2. Soda water – Solute is carbon dioxide and solvent is water.
3. Sugar solution – Solute is sugar and solvent is water.
Question 9. Common examples of colloids
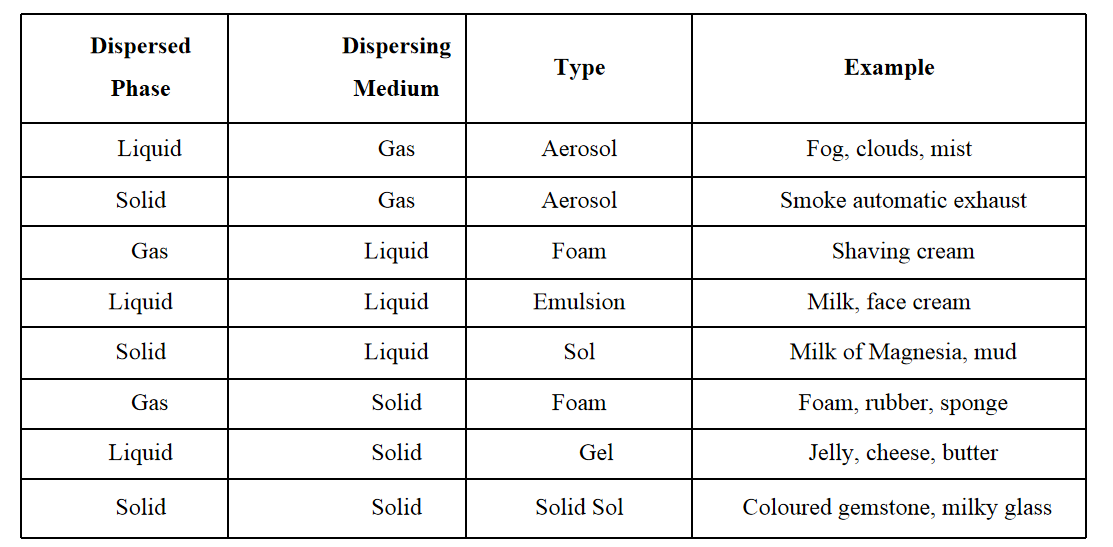
Question 10. Write the differences between physical and chemical changes.
Answer
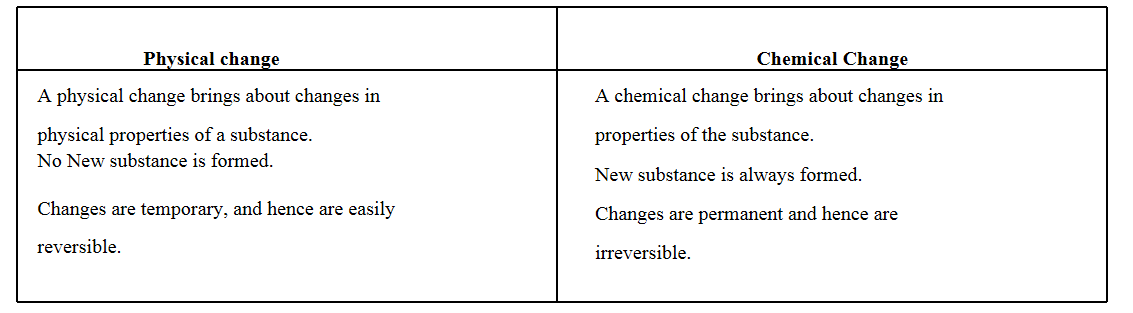
Question 11. Write the differences between physical properties of metals and non-metals.
Answer
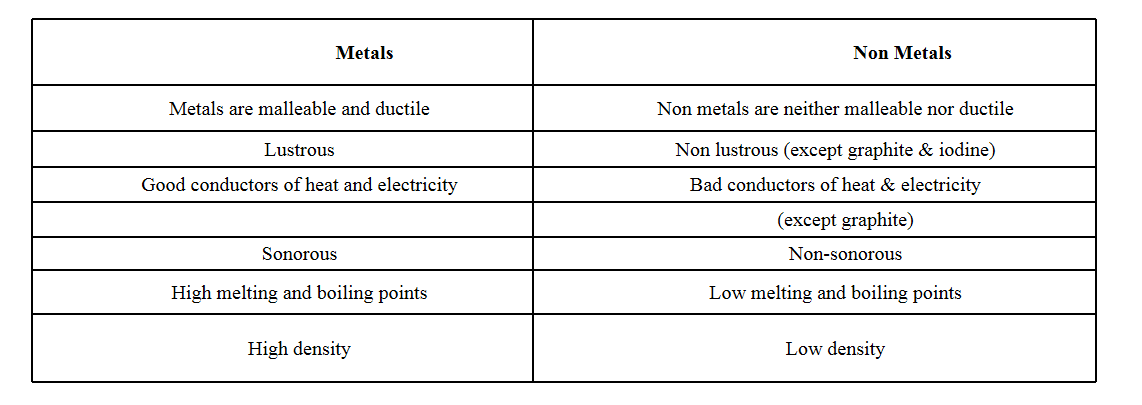
Question 12. A solution contains 50g of common salt in 350g of water. Calculate the concentration of solution.
Answer Mass of common salt(solute) = 50g
Mass of water(solvent) – 350g Total mass of salt + water(solution) = 400g
Mass by mass percentage of solution =
Mass of Solute x 100
Mass of Solution 50 100 = 12.5%
400
Is Matter Around Us Pure KSEEB Class 9 Detailed Solutions
Is Matter Around Us Pure High Order Thinking Questions
Question 1. Three Students A, B, and C prepared mixtures using chalk powder, common salt and milk respectively in water. Whose mixture:
1. Would not leave Residue on filter paper after filtration.
2. Would show Tyndall effect.
3. Would give clean or transparent solution.
4. Could be filtered by filter paper.
Answer.
1. Mixture of common salt and water.
2. Mixture of chalk powder with water and milk with water.
3. Mixture of common salt and water.
4. Mixture of chalk powder and water.
Question 2. Write your observations when the following process takes place.
1. Abeam of light is passed through a colloidal solution.
2. A saturated solution of potassium chloride prepared at 608°C is allowed to cool at room temperature.
Answer.
1. The path of a light becomes visible,
2. Potassium Chloride crystallizes out.
Question 3. Write the role of the following in water
purification.
1. Sedimentation tank
2. Loading tank
3. Chlorination tank
Answer
1. To allow solids to settle
2. To sediment the suspended impurities
3. To kill bacteria
Question 4. How is heating of sugar and heating of Ammonium Chloride different from each other?
Answer Ammonium chloride is a sublime, so it changes directly into gas on heating.
Sugar gets charred on heating to dryness.
Question 5. You are given two samples of water labelled as A and B. Sample A boils at 100°C and sample B boils at 102°C. Which sample of water will not freeze at 0°C? Comment.
Answer Sample B will not freeze at 0°C because it is not pure water. At 1 atm, the boiling point of pure water is 100°C and the freezing point of the pure water is 0°C.
Is Matter Around Us Pure Unit test Multiple choice questions
Question 1. Allows are______
- Pure substance
- Homogeneous mixtures
- Compounds
- Of fixed composition
Answer (2)
Question 2. Mixture o blue ink and red ink can be separated by_____
- Evaporation
- Separating funnel
- Chromatography
- Distillation
Answer (3)
Question 3. 1) Apure substance has fixed melting point
2) The properties of compound are similar to that of its components. Which is the correct statement______
- Both A and B are true statements
- A is true but B is false
- A is false but b is true
- Both A and B are false statements
Answer (2)
Question 4. The type of protein present in milk is______
- Casein
- Albumin
- Soya bean
- Keratin
Answer (1)
Is Matter Around Us Pure KSEEB Class 9 Question Answers
Question 5. 24 karat gold is______
- 100% pure gold
- 99%GOLD
- 95%Gold
- 91%GOLD
Answer (1)
Is Matter Around Us Pure Fill in the blanks
1. Distilled water is a pure substance.
2. Air is a mixture.
3. The liquids which do not mix are immiscible liquids.
4. Iodised salt is a heterogeneous mixture of sodium chloride and potassium Iodine.
5. The process used by milkmen to separate cream from milk is centrifugation.
6. The concentration of poisonous gases in air is expressed in parts per million ppm.
Is Matter Around Us Pure Answer the following one mark
Question 1. Is rainwater or distilled water a pure substance?
Answer Yes, because it contains particles of only water.
Question 2. Give an example of non-aqueous solution.
Answer Iodine in carbon tetrachloride.
Question 3. The particle size of a substance A present in water is 200 nm. What is the nature of the solution?
Answer Suspension
Question 4. How will you separate the fine mud particles floating in water?
Answer By coagulation – Using alum and then filtering.
Question 5. What is Rust?
Answer When iron is exposed to air and moisture for a few days, a brown colour substance called rust is formed. Chemically rust is hydrated oxide of iron.
Is Matter Around Us Pure Answer the following Two Marks
1. What happens when a saturated solution is
1. Cooled
2. Further heated
Answer
1. When saturated solution is cooled some of the dissolved solid separates out because solubility usually decreases with temperature.
2. When a saturated solution is heated, it can dissolve more of the solute because solubility usually increases with temperature.
Question 2. Give one example of
1. A solid foam and solid Sol
2. Liquid in a liquid solution
Answer
1. Solid foam – Sponge, Rubber
2. Solid sol – Milky glass, coloured gemstone Liquid and liquid solution – Vinegar (acetic acid- water).
Is Matter Around Us Pure Activity
Question 1.
Answer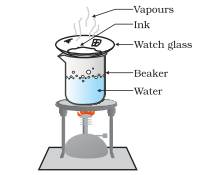
Method/Experimental procedure
Put a few drops of blue or black ink on a watch glass and place it on a beaker ha full of water as shown in figure. The water in the beaker is heated and the steam thus formed will in turn heat up the ink.
Observation: The water present in the ink on a watch glass will evaporate and ultimately a blue or black Residue will be left on the watch glass.
Conclusion: This method can be used to separate non-volatile components (solutes ) dissolved in volatile solvents by the method of evaporation.
Question 2.
Answer
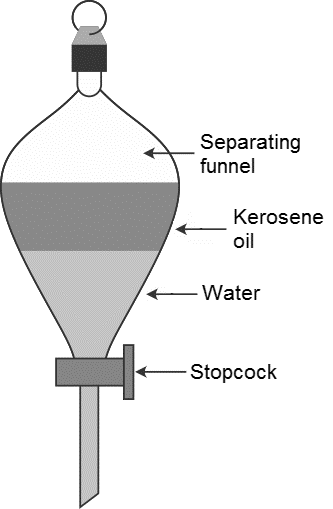
Method/Experimental procedure
Pour the mixture of kerosene oil and water in a separating funnel and keep undisturbed for some time so that separate layers of oil and water are fonned. Open the stop cock of the separating fimnel and pour out the lower layer of water carefully. Close the stop cock of the separating funnel as the oil reaches the stop cock.
Observation: Observe the setup carefully.
Conclusion: This technique is based on the principle that immiscible liquid separate out in layers depending on the densities.
Class 9 Science Chapter 2 KSEEB Textbook Solutions
Question 3.
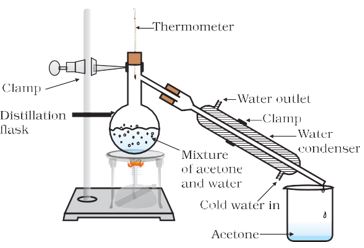
Answer
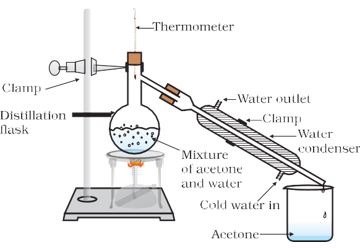
Method/Experimental procedure
The mixture of acetone and water in a distillation flask. Fit it with a thermometer. Arrange the apparatus as shown in figure. Heat the mixture slowly keeping a close watch at the thermometer.
Observation: The acetone vaporises and condenses in the condenser. Water is left behind in the distillation flask.
Conclusion: Thus, the separation of the liquid mixture into individual components can be achieved at their respective boiling points; the more volatile component distils over first, while the less volatile component distils over afterwards.
This technique is used to separate two miscible liquids by distillation.
Question 4. .
Answer
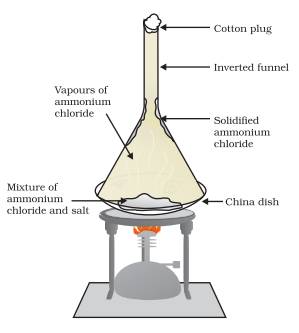
Method/Experimental procedure
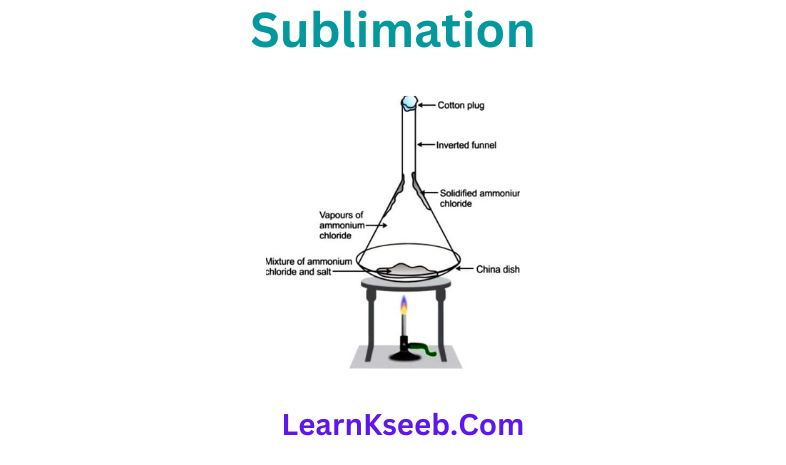
A mixture consisting of Ammonium Chloride and common salt is taken in a China dish and is covered with a perforated filter paper. An inverted funnel is then placed on the China dish and its stem is closed with a cotton plug. The dish is heated gently.
Observation: The ammonium chloride changes into vapour, these vapours papers pass through the perforations of the filter paper and then get deposited on the inner surface of the funnel. The common salt remains in the China dish as Residue.
Conclusion: Thus, the process of sublimation can be used to separate sublimable volatile components from the non volatile components of a mixture.
Question 5.
Answer
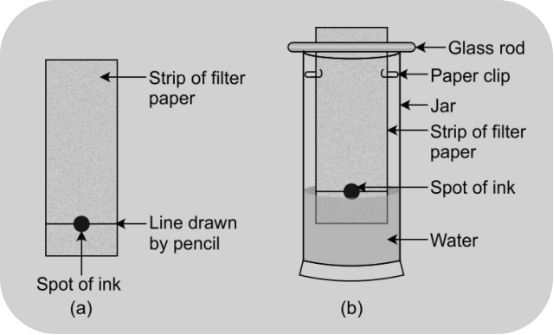
Method/Experimental procedure
Take a thin strip of filter paper. Draw a line on it using pencil, approximately 3 cm above the lower edge. Put a small drop of ink from a sketch pen or fountain pen at the centre of the line and let it dry. Suspend the paper in a glass jar containing water so that the spot of ink on the paper is just above the water level and leave it undisturbed.
Observation: As water rises up the paper by capillary action and flows over the spot, it takes along with it dye particles
Conclusion: The coloured component that is more soluble in water rises faster and in this way, the colours get separated.
This technique is used for separation of colours based upon the different solubilities in the same solvent
Question 6. Separation of two miscible liquids by fractional distillation.
Answer
Method/Experimental procedure
Take a mixture of two liquids chloroform and benzene in a distillation flask and boil the mixture. Set up the Apparatus as shown in figure and carry out experiment using a fractional column.
Observation: When the mixture is boiled, the vapours of less volatile liquid (benzene) condense more rapidly than the more volatile liquid (chloroform).
Conclusion: Vapours reach the top of the column and escape into the condenser, they consist entirely of the more volatile component i.e. chloroform will be collected in the flask.
Thus, separation of two miscible liquids whose is boiling points differ by less than 25 K can be achieved.