KSEEB 1st PUC Chemistry Equilibrium Short Answer Questions
Question 1. In the case of pure water or an aqueous solution, show that pH + pOH =14. Comment on whether this value will be greater than or less than 14 at 0°C and 100°C.
Answer:
We know, that pH+pOH = pKw and pKw= -log
The rise in temperature increases the value of Kw
Therefore, Kw(0 °C) < Kw(25°C)<Kw(100 °C)
Since pKw = -log10Kw,
pKw(100 °C)< pKw(25 °C) < pKw(0°C).
Now, at 25 °C, pKw= 14.
Therefore, pKw (100 °C) < 14 < pKw(0°C)
And pH + pOH(100°C) < pH + pOH(25°C) < pH+ pOH(0°C)
Hence, the value of (pH+ pOH) will be higher at 0°C than at 25 °C, but it will be lower at 100 °C than at 25 °C.
Question 2. At a certain temperature, the Kw of pure water =10-12. VVluit will be the pH -range, and pH of the neutral solution at that temperature.
Answer:
At the given temperature, Kw = 10-12. Hence, at that temperature, pKw= 12. We know that at a particular temperature, the pH -scale ranges from 0 to pKw and pH = \(\frac {1}{2} p K_w\)
For a neutral solution at that temperature. Therefore, at the given temperature, the range of the pH -scale will be from 0 to 12 and the pH of a neutral solution at that temperature will be
⇒ \(\frac {1}{2} p K_w=\frac{1}{2} \times 12=6\)

Short answer questions for Chapter 7 Equilibrium in 1st PUC Chemistry
Question 3. At a certain temperature, the ionization constants of two weak acids, HA and HB are Ka1 and’ ka2, respectively. If Ka1> Ka2 and the concentration of the aqueous solutions of both acids is 0.1(M), then which solution will have a higher pH?
Answer:
As the ionization constant of HA is higher than that of HB, the degree of ionization ofHA in its 0.1 M solution is higher than that of HB in its 0.1 M solution. As a result, the molar concentration of H3O+ions in 0.1(M) HA solution will be more than that in 0.1(M) HB solution. Therefore, the pH of the HB solution will be greater than that of the HA solution.
Question 4. The pH of a buffer solution remains almost unchanged even after dilution—explain.
Answer:
Let a buffer solution that consists of a weak acid (HX) and its salt (MX). For this buffer,
⇒ \(p H=p K_a+\log \frac{[\text { salt }]}{[\text { acid }]}\) At a certain temperature, the value of pKa is constant.
Hence, at a given temperature, the pH of the buffer solution depends upon the ratio of the molar concentrations of the salt to the acid in the solution. As the solution is diluted, this ratio remains the same. As a result, the pH of the solution remains unaltered.
Question 5. Consider the salts given below. For which salt(s) will the pH of the aqueous solution(s) be independent of the concentration of the salt? CH3NH3l, (NH4)3PO4, KCN and (NH4)2CO3.
Answer:
The pH of an aqueous solution of the salt of a weak acid and a weak base does not depend upon the concentration of salt. Among the given salts, (NH4)3PO4 and (NH4)2CO3 are produced from a weak acid and weak base. Hence, the pH of their aqueous solutions does not depend on the concentration of the salt.
Question 6. Write the correct order of increasing acid strength among HCO3< HSO3 < H3O+ < HSO3F.
Answer:
HSO3F is a super acid. So, its strength is the maximum among all the given acids. Comparing the dissociation constants of the remaining acids gives the order in terms of dissociation constant: Now, the larger the value of Ka for an acid, the higher the strength of the acid, So, the increasing order of acid strengths of the given acids will be HCO3< HSO3 < H3O+ < HSO3F.
Question 7. Under experimental conditions/ for the reaction, N2(g) + 3H2(g). 2NH3(g), tithe equilibrium mixture contains 17% of NH3 and 83% of (N2 + H2). Under the same conditions, what will be the percentages of NH3 and (N2 + H2) in the reaction 2NH3(g) N2(g) + 3H2(g) at equilibrium?
Answer:
Under similar experimental conditions, the same equilibrium will be established for a reversible reaction irrespective of whether the reaction is initiated with the reactants or the products. Thus for reaction, 2NH3(g)N2(g)+3H2(g), The same percentages of NH3 and (N2 + H2) will be present at equilibrium as in the case of the reaction N2(g) +3H2(g) 2NH2(g)
1st PUC Chemistry Chapter 7 Equilibrium Important Questions and Answers
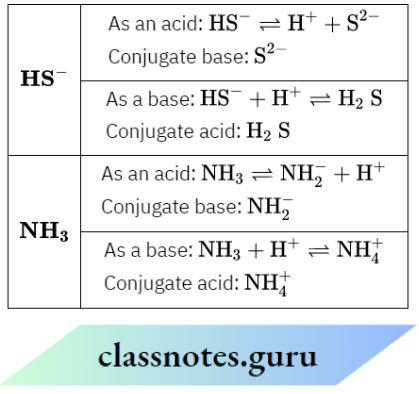
Question 8. Each HS– and NH3 can act as Bronsted acid and Bronsted base—why? Write the formula of conjugate base and conjugate acid in each case.
Answer:
According to the Bronsted-Lowry concept, an acid is a proton donor and a base is a proton acceptor. The given species can accept as well as donate protons, so they can act as both acid and base.
Question 9. The concentration of H3O+ ions in solution is 1000 times that in solution B. What is the difference between the values of pH of these two solutions?
Answer:
Given: [HgO+]a = 1000 × [H3O+]
Where [H2O+]A and [H3O+]B are the concentrations of H3O+ ions in solutions A and B respectively.
Therefore, pH (solution A)
= -log10[H3O+]A = -log(103×[H3O+]B
=- 3- log10[H3O+]B =- 3 + pH of solution B
pH of (solution B) – pH of (solution A) = 3
