Karnataka Class 9 Science Model Question Papers 2023 Set 6
Choose The Correct Alternative And Write The Complete Answer Along With Its Alphabet In The Sheet Provided:
Question 1. As the pressure of air decreases, the boiling point of the liquid.
- Decreases
- Increases
- Does not changes
- None of these
Answer: 1. Decreases
Question 2.50gm sugar is dissolved in a glass of water at 30″c, on heating this solution it will
- Crystallize
- Evaporate
- Become unsaturated
- Sugar will char
Answer: 3. Become unsaturated
Karnataka 9th Standard Science Model Paper 2023 Set 6 Free PDF with Solutions
Question 3. Fielder giving a swing while catching a ball is an example of
- Inertia
- Momentum
- Newton’s second law of motion
- Newton’s first law of motion
Answer: 3. Newton’s second law of motion

Question 4. The relative density of silver is 10.8 and the density of water is 103 kg/m2. The density of silver is
- 1.8 x104N/m3
- 10.8 x 103 N/m3
- 1.8 x 104 kg/m3
- 10.8 x 104 kg/m3
Answer: 4. 10.8 x 104 kg/m3
Question 5. Speed (s), wavelength (x), and frequency (v) of sound are related as
- s = u x v
- v = s x u
- u = s x v
- u = s/v
Answer: 3. u = s x v
Class 9 Karnataka Science Model Paper 2023 Set 6 Solved Questions PDF
Question 5. For the following chemical equation if the law of conservation of mass is correct, what would be the number of H2 molecules? N2+H2→ 2NH3
- 2
- 3
- 4
- 6
Answer: 2. 3
Question 7. Rutherford’s experiment on the scattering of alpha particles showed for the first time that the atom has
- Electrons
- Protons
- Nucleus
- Neutrons
Answer: 3. Nucleus
Question 8. Which of the following produces seeds without fruit
- Grapes
- Fern
- Paddy
- Cycas
Answer: 4. Cycas
Question 9. BCG vaccine is used to curb
- Pneumonia
- Tuberculosis
- Polio
- Amoebiasis
Answer: 2. Tuberculosis
Question 10. Plant breeding methods include
- Introduction of the crop
- Selection of desired traits
- Hybridization
- All of these techniques
Answer: 4. All of these techniques
Match the following:
A B
Waterborne disease keeps the body fit
Exercise contaminated water
Improves our lung capacity plasmodium
Malaria Relaxation
Answer:
A B
Waterborne disease contaminated water
Exercise keeps the body fit
Improves our lung capacity Relaxation
Malaria plasmodium
Answer The Following:
Question 1. State the numerical value of the Avogadro constant
Answer: Avogadro’s constant = 6.022 x 1023
Question 2. Find the number of electrons, protons, and neutrons possessed by the alpha particles \(\left({ }_2^4 \mathrm{He}^{++}\right)\) used in the gold lead experiment
Answer: E = Nil, P = 2, N = 2
Question 3. Name any two groups of microorganisms from which antibiotics can be extracted.
Answer: Fungi and bacteria
Question 4. Who discovered the neutron?
Answer: James Chadwick
Question 5. How many joules make a one-kilowatt hour?
Answer: 1kwh = 3.6 x106j
Question 6. What is reverberation?
Answer: Reverberation is the repeated reflection of sound waves that results in the persistence of sound for some time.
Question 7. When we jump into a swimming pool, we feel lighter, why?
Answer: This is because upthrust acts on us and our apparent weight is less than the actual weight
Question 8. What is immunization?
Answer: Immunization is the process of rendering our body immune to certain diseases through vaccination.
Karnataka Board 9th Science Model Paper 2023 Set 6 Latest Exam Pattern PDF
Question 9. Define the atomic mass unit.
Answer: Atomic mass unit is exactly equal to 1/12 th the
mass of one atom of carbon – 12
Question 10. Write the notation of an atom ‘X’ if the mass number is A and the atomic number is Z.
Answer: zXA
Answer The Following:
Question 1. Convert the following temperatures to the Celsius scale
- 293K
- 470K
Answer:
- 293 – 273
= 20C° - 470 – 273
=197C°
Question 2. Mention the properties of a solution.
Answer:
- A solution is a homogenous mixture.
- The particles are smaller. So they cannot be seen by the naked eye.
- They do not scatter a beam of light passing through the solution.
- The solute particles cannot be separated and the particles do not settle down.
Question 3. Calculate the molecular mass of K2CO3, given atomic mass K = 39u, C = 12u and 0 = 16u
Answer:
The molecular mass of K2CO3
=K x 2 + C x 1 + 0 x 3
= 39 x 2+ 12 5 1 + 16 x3
= 78+12 + 48
= 138u
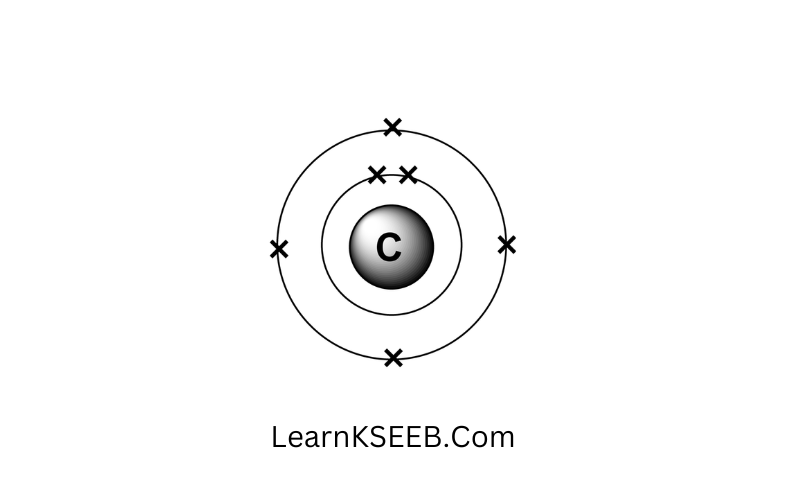
Question 4. Write the distribution of electrons in carbon
(z = 6) and sodium (z -11) atoms.
Answer: C = 6 Na= 11

Question 5.Define
- Endocytosis
- Plasmolysis
Answer:
- The flexibility of the cell membrane enables the cell to engulf food and other material from its external environment. Such processes are known as endocytosis.
- when a living plant cell loses water through osmosis there is shrinkage or contraction of the contents of the cell away from the cell wall is known as plasmolysis.
Question 6. Mention the functions of the Golgi apparatus.
Answer:
- Its functions include the storage, modification, and packing of products in vesicles.
- complex sugars may be made from simple sugars in the Golgi apparatus.
Question 7. Striated muscles are also called voluntary muscles. Why?
Answer: Since these muscles are under our control and present in our limbs and hands
Question 8. Name different types of meristematic tissue present in the plant body.
Answer:
- Apical meristem
- Intercalary meristem
- lateral meristem
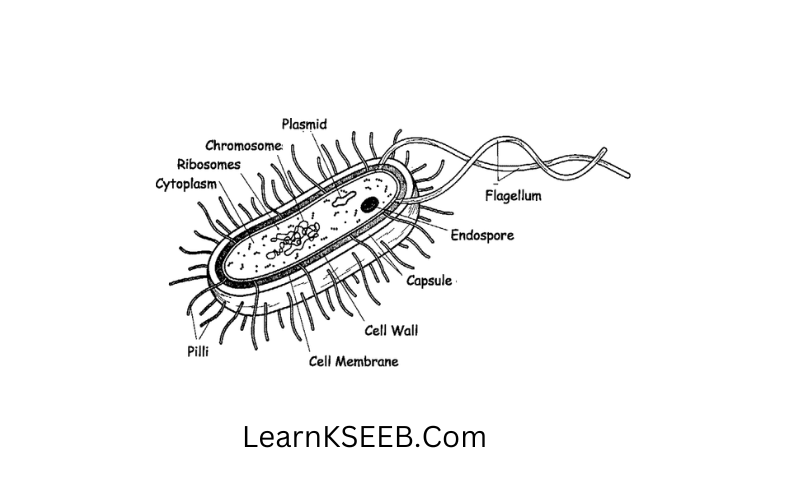
Question 9. Draw a diagram of bacteria
Answer:
Question 10. Mention the classes of vertebrates.
Answer: Pisces, amphibia, reptilia, aves, and mammals.
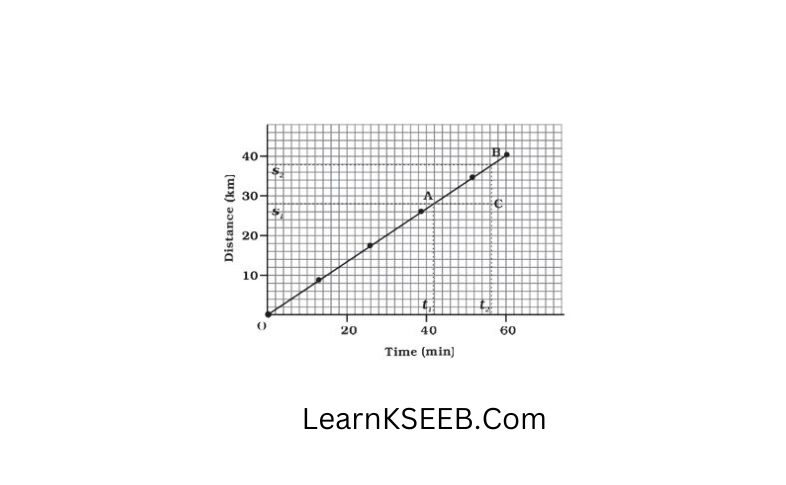
Question 11. Draw a distance-time graph of an object moving with uniform speed.
Answer:
 Question 12. Derive an equation for position time relation.
Question 12. Derive an equation for position time relation.
Answer:
Let us consider that the object has traveled a distance ‘s’ in time ‘t’ under uniform accelerated ‘a’
Thus distance ‘s’ traveled by the object is given by
s = area OABC
= area of the rectangle OADC + area of the triangle ABD
= OA OC + 1/2 (AD BD)
substituting OA-u, OC = AD = t and BD = at
S = u x t + \(\frac{1}{2}\) t x at
∴ S = u + \(\frac{1}{2} a t^2\)
Question 13. Which has more inertia a rubber ball and a stone of the same size?
Answer: mass of a stone is more than the a rubber ball. Hence inertia of the stone is greater than that of a rubber ball.
Question 14. A boy of mass 50kg runs up a staircase of 45 steps in 9sec. If the height of each step is 15cm. Find his power. Take g = 10msA
Answer:
weight of the body
mg = 50kg x 10ms-2
= 500 N
Height of the staircase
h = 45 x \(\frac{15}{100}\)
= 6.75m
Time is taken to climb t = 9s
P = 375w
Question 15. A battery lights a bulb. Describe the energy changes involved in the process.
Answer: A fully charged battery has chemical energy which converts to electrical energy which eventually converts into heat and light energy.
chemical → Electrical → Heat → Light energy
Question 16. Give two practical applications of the reflection of sound waves.
Answer:
- It is used to measure the distance and speed of underwater objects.
- working of a stethoscope!s also based on the reflection of sound.
Question 17. How do we kill microbes that enter our bodies?
Answer: We kill these disease-causing microbes with the help of medicines that block the microbial synthesis pathways of microbes.
Question 18. How does the atmosphere act as a blanket?
Answer:
- It keeps the average temperature of the earth fairly constant during day time and even during the course of the whole year.
- It prevents a sudden increase in the temperature during day time.
- It slows down the escape of heat from the surface of the earth.
Question 19. Name any two weeds
Answer: Parlhemum and xanthium.
Answer The Following :
Question 1.
- What is ultrasound? Name two animals that produce ultrasound
- How are moths of certain families are able to escape capture from bats?
Answer:
- Frequencies higher than 20KHZ are called ultrasound Ex: Dolphins, bats
- These moths can hear the high-frequency squeaks of the bat and know when a bat is flying nearby and are able to escape capture.
Karnataka State Board Class 9 Science Model Paper 2023 Set 6 with Step-by-Step Solutions
Question 2. How can we prevent infectious diseases?
Answer:
- By providing living conditions that are not overcrowded we can prevent airborne diseases.
- By providing clean drinking water. We can prevent waterborne diseases.
- Vector-borne diseases can be prevented by keeping our surroundings clean.
Question 3.
- Name the term used for unwanted plants in a cultivated Held and give one example.
- Why do farmers remove them from crop fields?
- Mention one control measure by which these can be destroyed.
Answer:
- Weeds
Example: Xanthium - They extract the nutrients from the soil that are meant for the crop plant.
- By manual weeding or by applying weedicides.
Question 4.
- What is sublimation? Give an example
- What happens when acetone is poured on the palm
Answer:
- The change of a solid directly into the gaseous state without passing through the liquid state is known as sublimation. Example: Naphthalene, ammonium chloride
- It will evaporate taking the latent heat of vaporization from the palm.
Answer The Following:
Question 1.
(1) State two characteristic properties of each of the following
- Solid
- Liquid
- Gas
(2) Shiva dropped a crystal of KMno4 in two beakers A and B containing hot water and cold water respectively. After keeping the beakers undisturbed for some time what did he observe and why?
Answer:
(1) Solid:
- They have fixed shapes and volume
- They are rigid
- Liquid
- They have fixed volume and no mass
- They are fluid
Gas:
- They have no fixed shape or volume
- They are highly compressible
(2) KMnO4 crystals diffuse faster in hot water because as the temperature increases diffusion increases
Question 2.
(1) State two characteristic features of vertebrates
(2) State reasons for the following
- Echidna and platypus lay eggs, but are considered mammals.
- The forelimbs of birds are modified.
Answer:
(1)
- They have a notochord.
- The true body cavity is present.
(2)
- They have mammary glands to feed their young ones.
- Reduced body weight for flight.
9th Standard Karnataka Science Sample Paper 2023 Set 6 Important Questions PDF
Question 3. Explain Rutherford’s particle scattering experiment observations and conclusions drawn from this experiment.
Answer:
Observations:
- Most of the fast-moving a – particles passed straight through the gold foil.
- Some of the a – particles were deflected by the foil’s small angles.
- One out of every 12000 particles appeared to rebound.
Conclusions:
- Most of the space inside the atom is empty because most of the a – particles passed through the gold foil without getting deflected.
- Very few particles were deflected from the path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of a – particles were deflected by 180°, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
Question 4. What is the work to be done to increase the velocity of a car from 30kmh to 60kmh 1 if the mass of the car is 1500kg?
Answer:
Mass of the car m = 1500kg
The initial velocity of car u = 30km-1
similarly, the final velocity of the car
v = 60kmh-1
\(=\frac{50}{3}\)ms-1
∴ The initial kinetic energy of the car
Eki = \(\frac{1}{2}\) mu2
\(\begin{aligned}& =\frac{1}{2} \times 1500 \times\left(\frac{25}{3}\right)^2 \\
& =\frac{156250}{3}=52083
\end{aligned}\)
The final kinetic energy of the car
\(\mathrm{E}_{\mathrm{kf}}=\frac{1}{2} \times 1500 \times\left(\frac{50}{3}\right)^2\) \(\frac{625000}{3}\)= 208333
Thus the work done = change in kinetic energy
= Ekf- Eki
= 208333 – 52083
= 156250J
