KSEEB Solutions For Class 9 Science Chapter 4 Structure Of The Atom Important Concepts
Structure of an atom, electrons, protons and neutrons. Atomic number and mass number, isotopes and isobars.
Atom
Atom is not the smallest indivisible particle but is made up of still smaller particles, i.e, electrons, protons and neutrons called fundamental particles are subatomic particles.
Proton
The fundamental particle which carries one nit positive charge and has mass nearly to that of hydrogen atom.
KSEEB Solutions for Class 9 Science Chapter 4
Electron
The fundamental particle which carries one unit negative charge and has mass nearly to hydrogen atom.
\(=\frac{1}{1840} \text { th of } \mathrm{H} \text { atom }\)
Read and Learn More KSEEB Solutions for Class 9 Science
Neutron
The fundamental particle which is neutral but has mass nearly equal to that of a proton.
Discovery of Subatomic particles
Electron -J. J Thomson Proton – Goldstein
Neutron – Chadwick
Atomic number(Z)
It is equal to the number of protons present in the nucleus.
| Class 9 Social Science | Class 9 Science | Class 9 Maths |
Mass number(A)
It is equal to the sum of protons and neutrons.
Rutherford’s model of an atom
- An extremely small positively charged part at the centre called the nucleus in which the entire mass of an atom is concentrated.
- A number of negatively charged electrons surround ing the nucleus at relatively large distance from it. The number of electrons is equal to the net positive charge on the nucleus.
- Nucleus and electrons are held together by the strong electrostatic forces of attraction.
Thomson’s model of an atom
- Inside the atom electrons are present.
- These electrons are embedded in spheres of positive charge.
- Mass of an atom is due to electrons only.
- The negative and positive charges balance each
- Atom as a whole is neutral.
KSEEB Class 9 Science Structure of the Atom Solutions
Bohr’s model of an atom
- An atom consists of a positively charged centre called the nucleus.
- Electrons revolve in orbits with well defined energy
- Electrons moving in the same orbit will not lose or gain energy.
- Supply of energy exits the electrons to high energy
Bohr-Bury Scheme
- First shell can have maximum 2, second shell can have maximum 8, third shell can have 18 electrons and 4th shell can have 32 electrons.
- Maximum number of electrons which can be ac commodated in an energy level of an atom is given by formula is 2n(n-The number of energy level)
Isotopes
Isotopes are the atoms of the same element possessing different mass numbers but the same atomic number.
Example: Chlorine
40Ar18 40K19
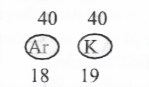
Isobars
The atoms of different elements, havingsome mass
number but different atomic numbers
Example: Argon, Potassium have same mass number but different atomic numbers.
Representation of atomic and mass number of an element.
A – Mass number
X – Symbol of the element
Z – Atomic number.
Example: 16 – Mass number l110
O – Oxygen element
8 – Atomic number
Structure Of The Atom Exercises
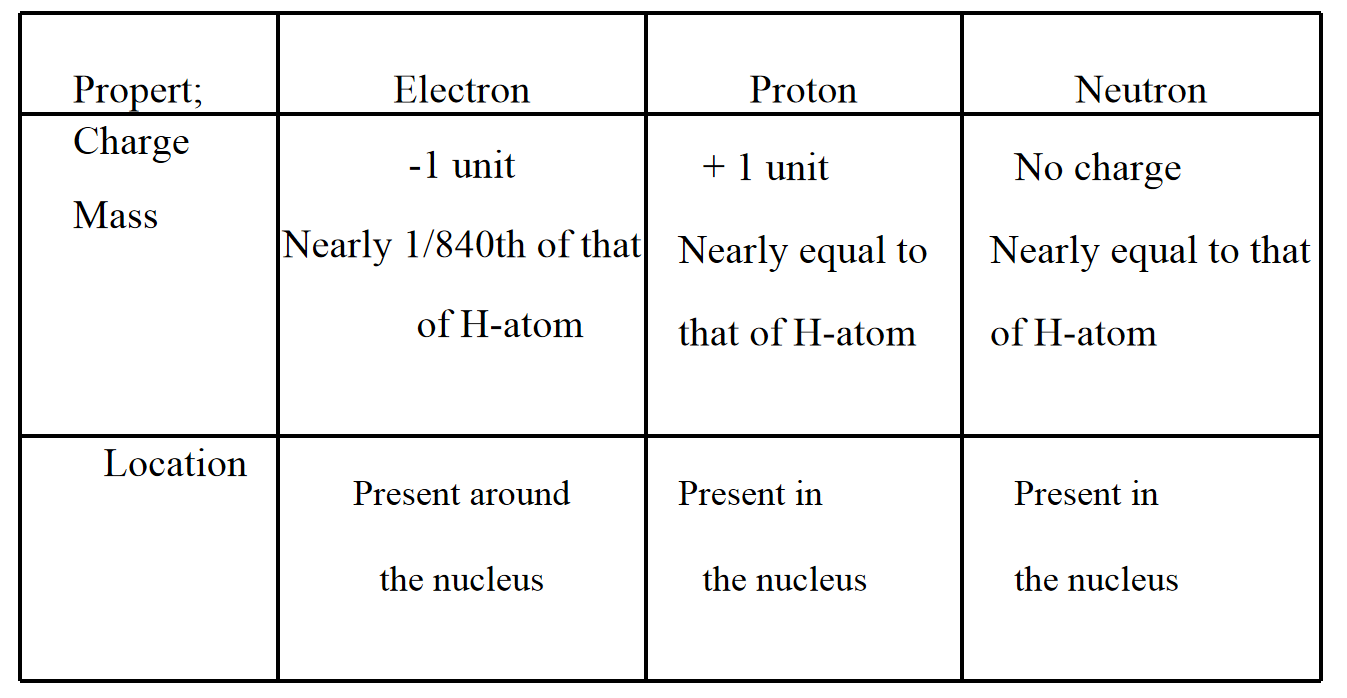
1. Compare the properties of electrons protons and neutrons.
Answer:
2. What are the limitations of JJ Thomson’s model of the atom?
Answer: J. J. Thomson’s model could not explain the re¬ sults of the scattering experiment carried out by Rutherford.
3. What are the limitations of Rutherford’s model of the atom?
Answer:
- Any charged particle when accelerated is ex¬ expected to radiate energy.
- To remain in a circular orbit, the electron would need to undergo acceleration. Therefore, it would radiate energy.
- The loss of energy would lead to shrinking of the size of the orbit. Inshort time it would hit the nucleus. Therefore an atom cannot be expected to be stable
Karnataka Board 9th Science Chapter 4 Notes PDF
4. Describe Bohr’s model of the atom.
Answer: Bohr put forward the following postulates about the model of an atom.
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy.
These orbits or cells are called levels and are represented by the letters K, L, M, N

5. Compare all the proposed models of an atom given in the chapter.
Answer:
J. J. Thomson’s model of the atom
JJ Thomson recognized the subatomic particles, i.e, electron and proton but failed to explain that how they are arranged within the atom and how they are shielded electrically.
Rutherford’s model of the atom
Rutherford’s model of the atom proposed that a very tiny nucleus is present inside the atom and electron revolve around the nucleus. The stability of that atom could not be explained by this model. Neil Bohr’s model of the atom
Class 9 Science Chapter 4 KSEEB Solutions PDF
Neil bohr model of the atom
was more successful. He proposed that electrons are distributed in different shells with discrete energy levels around the nucleus. If the atomic shells are complete, then the atom will be stable and less reactive.
6. Summarise the rules for writing of distribution of electrons in various shells for the first 18 elements.
Answer: The number of electrons in different shells are fixed. The maximum number of electrons in any shell is given by the formula 2n2 where n is the number of shell as counted from the nucleus.Maximum number of electrons in 1st shell
(K shell) = 2n2= 2x 12= 2
Maximum number of electrons in 2nd shell
(L shell) = 2n2= 2*22= 8
Maximum number of electro ns in 3rd shell
(M shell) = 2n2=2×32= 18
Maximum number of electrons in 4th shell
(N shell) = 2n2=2×42= 32
7. Define valency by taking examples of silicon and oxygen.
Answer: Valency is defined as the number of electrons which an atom can lose or gain or share with other atoms so as to complete its octet. i.e, 8 electrons in the outermost shell.
Examples
1. Silicon
A tomic number of silicon =14
Number of electrons present =14
Electronic configuration= K – 2, L – 8, M – 4.
Thus, outermost shell has 4 electrons which it can share with other atoms to complete its orbit. Hence its valency = 4.
2. Oxygen
A tomic number of oxygen = 8
Number of electrons present = 8
Electronic configuration = K – 2, L – 6
Thus, outermost shell has 6 electrons. It will give two electrons to complete its octet. Hence valency = 2
8. Explain with examples
- Atomic number
- Mass number
- Isotopes
- Isobars
Structure Of The Atom Give any two uses of isotopes.
Answer:
1. Atomic number: The total number of protons present within the nucleus of an atom is known as atomic number.
Example: Sodium atom has 11 proton in its nucleus, therefore its atomic number is 11.
2. Mass number: The sum total of the masses of all the nucleons present in the nucleus of an atom, i e, number of protons and number of neutrons is called its mass number.
Example: Sodium atom has 11 protons and 12 neutrons in its nucleus, therefore its mass number is 11 + 2 = 23.
3. Isotopes: Atoms of the same element that have same atomic number but different mass numbers. Eg: Hydrogen has three isotopes.
\({ }_1^1 \mathrm{H} \quad{ }_1^2 \mathrm{H} \quad{ }_1^3 \mathrm{H}\)
The atomic number is 1 but mass numbers are 1,2,3.
4. Isobars: Atoms of different elements having same mass number but different atomic numbers. Eg: Calcium and Argon atoms have same mass number 40 but different atomic numbers 20 and 18.
18Cl40 20Ar40
9. Na+ has completely filled K and L shells. Explain.
Answer: Atomic number ofNa =11, i.e, it has 11 electrons. According to Bohr – Bury scheme
1st shel; (n =1) i.e, K – Shell have maximum electrons = 2n2 = 2x 12 = 2
2nd shell (n =2) i.e, L – Shell have maximum electrons = 2n2 = 2×22 = 8
The remaining one electron will enter into the 3rd shell. Thus, K and L shells are completely filled.
KSEEB 9th Science Chapter 4 Important Questions
10. If bromine atom is available in the form of say two isotopes.
35 Br 79 (49.7%) 35 Br 81 (50.3%)
Calculate the average atomic mass of bromine atom.
Answer: The percentage of Br isotope with mass number
79 = 49.7
The percentage of Br isotope with mass number
81 = 50.3
Average atomic mass=\(\frac{49.7 \times 79 \times 50.3 \times 81}{100}\)
\(=\frac{3926.3+4074.3}{100}\)
= 39.263 +40.743 = 80.006 u
11. Average atomic mass of sample of an element X is 16.2u. What are the percentages of isotope 16/8x &18/8x in the sample?
Answer: The percentage of 16/8x be the “A”
Then the percentage of 18/8x will be = 100 – A
That means
\(\frac{\mathrm{A} \times 16+(100-\mathrm{A}) 18}{100}=16.2\)
16A+1800-18A=1620
-2A=-180
\(A=\frac{-180}{-2}=90\)
This suggests that, a percentage of 16/8x = 90
and therefore, percentage of
18/8x = 100-90= 10
12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer: Element with Z = 3 is Lithium(Li)
Electronic configuration = \(\mathrm{K} \mathrm{L}
21\)
Thus, outermost shellhas 1 electron.
Hence, its valency = 1
13. Composition ofthe nuclei ofthe two atomic species X and Y are given as under:
\(\begin{array}{lll}
& X & Y \\
\text { Protons }= & 6 & 6 \\
\text { Neutrons }= & 6 & 8
\end{array}\)
Give the mass number of X and Y. What is the relationship between the two species?
Answer: Mass number = Number of protons + Number
of neutrons.
.’. Mass number ofX = 6 + 6=12
Mass number of Y = 6 + 8=14
As both contain the same number of protons i.e, 6, but they have different mass numbers i.e, 12 and 14. Hence, they are isotopes
14. Following statements, write T for true and F for false.
1. JJ Thomson proposed that the nucleus of an atom contains only nucleons. False
2. A neutron is formed by an electron and a proton combining together, therefore it is neutral. False
3. The mass of an electron is about 1/2000 times that of proton. True
4. An isotope of iodine is used for making tincture Iodine, which is used as medicine. False
15. Rutherford’s alpha-particle scattering ex¬ periment was responsible for the discovery of
- Atomic nucleus
- Electron
- Proton
- Netutron
Answer: 1. Atomic nucleus
16. Isotopes of an element have
- The same physical properties
- Different physical properties
- Different number of neutrons
- Different atomic numbers
Answer: 3. Different number of neutrons
17. Number of valence electrons in Cl ion are
- 16
- 8
- 17
- 18
Answer: 2. 8
18. Which of the following is a correct electronic configuration of sodium?
- 2,8
- 8, 2, 1
- 2, 1, 8
- 2, 8, 1
Answer: 2. 2, 8, 1
KSEEB Class 9 Science Textbook Solutions Chapter 4
19. Complete the following table

Structure Of The Atom Textual Questions
1. What are canal rays?
Answer: The positively charged radiation produced in a discharge tube containing a gas at low pressure when a high potential difference is applied between the electrodes are called canal rays
2. If an atom contains one electron and one pro¬ ton will it carry any charge or not?
Answer: No, it will not carry any charge because electron carrier’s one unit negative charge whereas proton carries one unit positive charge. The net charge
on the atom will therefore be zero.
3. On the basis of Thomson’s model of an atom explain how the atom is neutral as a whole.
Answer:
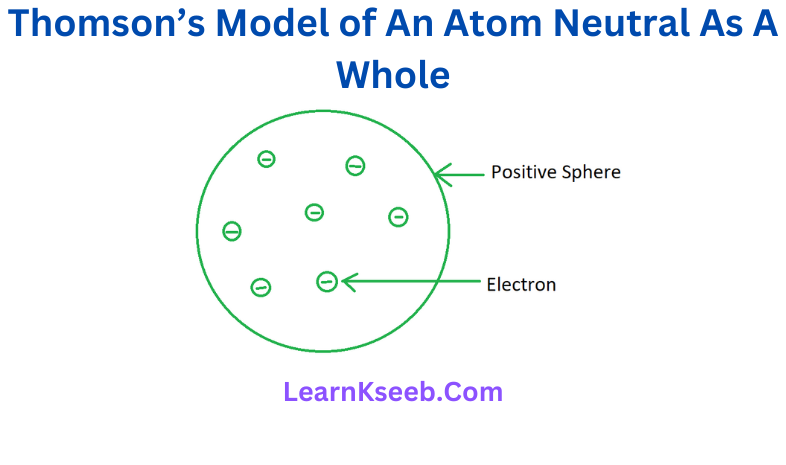
Thomson’s Model of an atom.
According to Thomson’s model, the atom is considered as a sphere of positive charge in which negatively charged electrons are embedded like
seeds in a watermelon. The total negative charge on the electrons is equal to the total positive charge on the sphere. Hence, the atom as a whole is electrically neutral.
4. On the basis of rutherford’s model of an atom, which some subatomic particle is present in the nucleus of an atom?
Answer: Protons
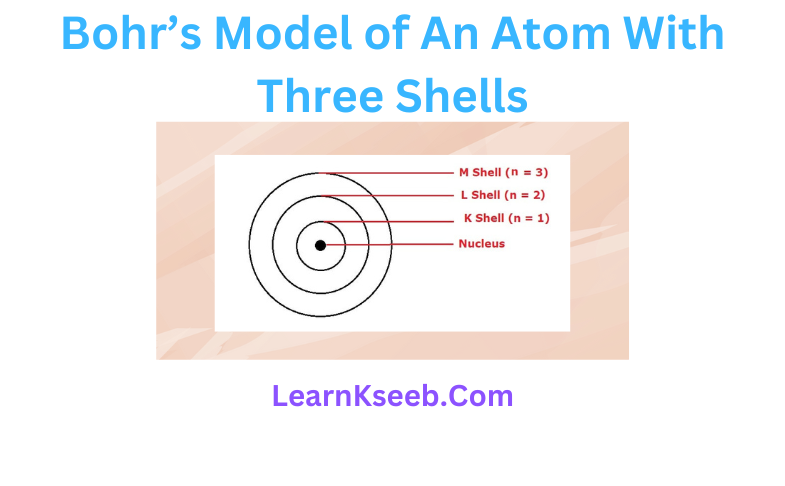
5. Draw a sketch of Bohr’s model of an atom with three shells
6. What do you think would be the observation if the a-particle scattering experiment is carried out using a foil of a metal other than
gold?
Answer: With the foil of any heavy metal like gold, ex¬ ample: platinum, silver, etc, the observation will be same but with the fall of a light metal, example lithium, the massive a -particles may push the nucleus, and may not be deflected back.
7. Name the three subatomic particles of an atom.
Answer: Electron, proton, and neutron.
8. Helium atom has an atomic mass of 4u and
two protons in its nucleus. How many neu¬
trons does it have?
Answer: Mass number(atomic number) = 4
Number of protons = 2 i.e, Atomic number = 2
Number of neutrons = Mass number – Atomic
number = 4 -2 = 2
Karnataka Board 9th Science Chapter 4 MCQs
9. Write the distribution of electrons in carbon
and sodium atoms.
Answer: For carbon atoms, Atomic number ofC = 6
Number of electrons = Atomic number = 6
Electronic distribution= K/2 L/4
For sodium atom, Atomic number ofNa =11
Number of electrons =Atomic number = 11
Electronic distribution = K /2 L/8 M/1
10. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: K-shell(n= 1) = 2 x 12 = 2
L shell (n = 2) = 2 * 22 = 8
When K and L shells are full, total number of electrons in the atom = 2 + 8 = 10
11. How will you find the valency of chlorine, sulphur, and magnesium?
Answer: Chlorine
Atomic number of chlorine =17
Electronic configuration = K, L, M
2 8 7
Valency of chlorine = 8-7=1 Sulphur
Atomic number of Sulphur = 16
Electronic configuration = K, L, M
2 8 6
Valency of sulphur =8-6 = 2
Magnesium
Atomic number of Magnesium = 12
Electronic configuration = K, L, M
2 8 2
Valency of magnesium is the number of valence electrons in the valence shell = 2.
12. If number of electrons in an atom is 8 and number of protons is also 8, then
1. What is the atomic number of the atom?
2. What is the charge of the atom?
Answer:
1. Atomic number = Number of protons = 8
2. As number of protons is equal to the number
of electrons, the atom will be neutral i.e, there is
no charge on the atom.
13. With the help of table find out the mass number of oxygen and sulphur atom.
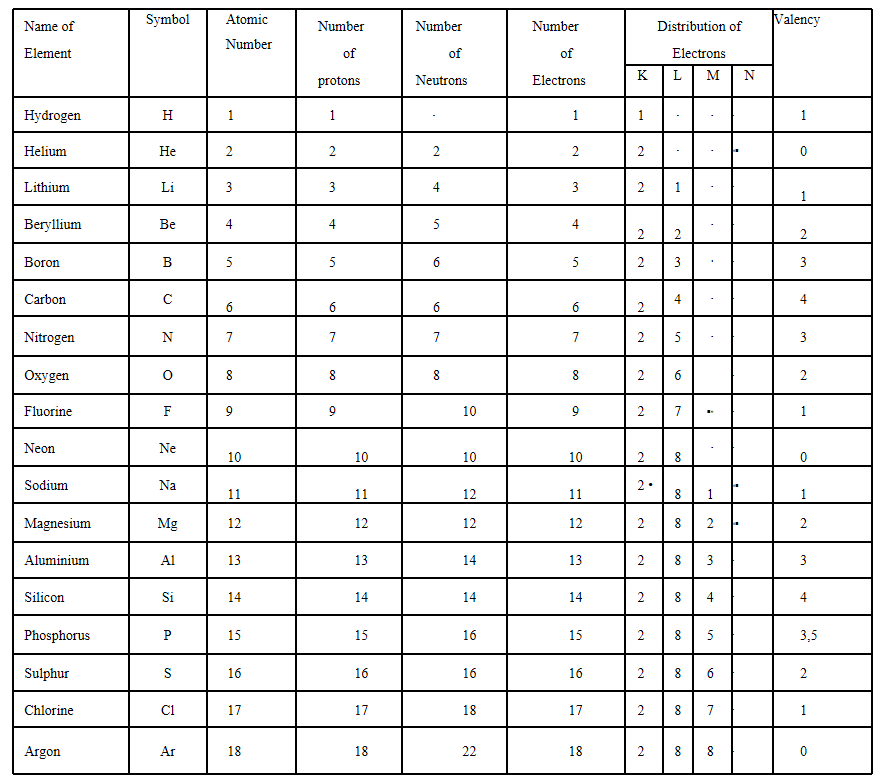
Answer: Oxygen atom
Atomic number = 8
Number of protons = 8
Number of neutrons = 8
Number of oxygen = Number of protons + Number of neutrons
Sulphur atom
Number of protons = 1 6
Number of neutrons =16
Mass number of sulphur = Number of protons + Number of neutrons = 16 + 16 = 32u
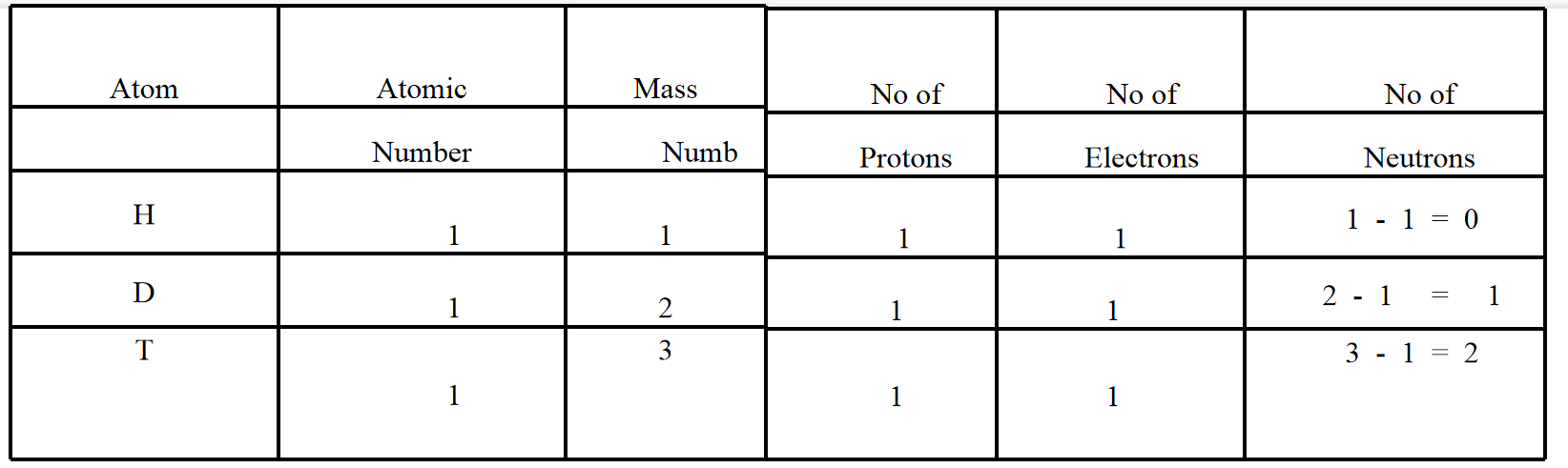
14. For the symbol, H.D. and T tabulate three subatomic particles found in each of them.
15. Write the electronic configuration of any one pair of isotopes and isobars.
Answer: Isotopes of chlorine = \(\text { Isotopes of chlorine }=\begin{array}{cc}
35 & 37 \\
17 & { }_{17}^{3 l}
\end{array}\)
Electronic configuration of each of
= K L M 2
8 7 40
Isobars:\({ }_{18}^{40} \quad \text { Ar }_{20}^{40} \mathrm{Ca}\)
electronic configuration of Ar = K L M
2 8 8
Electronic configuration of Ca=K L M N
2 8 8 2
Structure Of The Atom Additional Questions
1. Name the particles which determine the mass of an atom.
Answer: Proton and neutron
2. Write any two observations which support the fact that atoms are divisible.
Answer: Discovery of electrons and protons.
3. Why do Helium, Neon and Argon have a zero valency?
Answer: Helium has 2 electrons in its only energy shell, while argon and neon have 8 electrons in the valence shells. As these have maximum number of electrons in the valence shells, theydo not have any tendency to combine with other elements. Hence, they have a valency equal to zero.
4. Find out the valency of element X having atomic number 16.
Answer: For element X
Atomic number = 16
Electronic configuration =2, 8, 6
Valency=8-6 = 2
5. List applications ofany three isotopes in various fields.
Answer:
1. An isotope of Uranium is used as a fuel in nuclear reactors.
2. An isotope ofCobalt is used inthe treatment of cancer.
3. An isotope ofiodine is used in the treatment of goiter
6. What do you mean by excited state of an atom?
Answer: When the electron in the atom absorbs energy and jumps to some higher orbit it is called excited state
8. Give examples from everyday life where we use cathode ray tubes.
Answer:
1. Television picture tube
2. Fluorescent light tubes or bulbs
9. What do you mean by
1. Valence electrons
2. Valence shell
Answer:
1. Valence electrons: The electrons present in the outermost shell ofan atom.
2. Valence shell: The outermost shellofan atom
10. What are nucleons?
Answer: Protons and neutrons present in the nucleus are
collectively called nucleons
1. Calculate the total number of electrons present in 1 mole of Methane.
Answer: Atomic number of C = 6. Thus, it has 6 electrons. Atomic number of H = 1. Thus, each H atom has one electron.
Number of electrons present in 1 molecule of
\(\mathrm{CH}_4\) = 6 + 4 = 10
Number of molecules in 1 mole of \(\mathrm{CH}_4\)
= 6.022 x \(10^{23}\)
Hence, 1 mole of will contain electrons
= 10×6.022 x \(10^{23}\)= 6.022 x \(10^{24}\)
2. Why did Rutherford choose gold and not another light element for his experiment?
Answer: Rutherford use gold for his experiment because he wanted as thin layer as possible for the experiment and gold foil was only about 1000 atom thick
3. Why atomic number is considered as better fundamental attribute of an element? State the reason.
Answer: Because elements are defined by the number of protons they possess. Chemical properties of an element change with the change in atomic number.
4. An ion Z2+ contains 18 electrons and 20 neutrons. Calculate the atomic number and mass number of element Z. Name the element Z.
Answer: Number of electrons in Z2+ ion = 18
Since positive ion is formed by the loss of electrons from the neutral atom and the number of electrons lost is equal to the number of units of positive charge on the ion.
Number of electrons in the neutral atom
= 18 + 2 = 20
Now, for a neutral atom,
Atomic number = Number of protons = Number of electrons
Thus, make number of element Z = 20
Mass number of element Z = Number of protons
+ Number of neutrons = 20 + 20 = 40
The given element Z with atomic number 20 is Calcium.
Structure of the Atom Class 9 KSEEB Question Answer
5. A student state that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with this statement? Justify your answer.
Answer: No, statement is incorrect. In an atom the number of protons and electrons is always equal.
6. How can you justify that protons are constituents of all atoms?
Answer: The mass and charge of the nucleus of the atom of any element are found to be whole number multiple of the mass and charge of proton. This shows that protons are present in all the atoms.
7. Give one application where electron beam is used.
Answer: Cathode rays (beam of electrons) when focused on a heavy metal like tungsten, molybdenum, etc, produce X – rays which find a wide application in medical field.
Structure Of The Atom Unit Test Multiple Choice Questions
1. Rutherford’s Alpha particle scattering experiment resulted into discovery of_______
1. Electron
2. Proton
3. Nucleus in the atom
4. Atomic mass
Answer: (3)
2. Elements with valency 1 are______
1. Always metals
2. Always metalloids
3. Either metals or nonmetals
4. Always nonmetals
Answer:(3)
3. Which of the following are true for an element?_______
a. Atomic number = number of protons + number ofneutrons
b. Mass number = number of protons + number of neutrons
c. Atomic mass = number of protons = number of neutrons
d. Mach number = number of protons = number of electrons
- 1&2
- 1&3
- 2&3
- 2&4
Answer: (4)
Structure Of The Atom Fill in the blanks
1 . Helium has 2 Valence Electrons and its valency is zero.
2. The heaviest particle in an atom is Neutron
3. The mass number is equal to number of neutrons
4. 2nd energy level can have maximum 8 electrons.
Structure Of The Atom State true or false
1. Cathode rays travel in straight line. True
2. Neutrons does not have any charge. True
3. Cathode rays are positively charged. False
Structure Of The Atom Answer the following
1. What is the mass of an electron?
Answer: \(\frac{1}{1836}\)of1 atomofH = 9.1x 1031kg 1836
2. Which isotope is used to detect blood clots?
Answer: Na – 24
3. Which of the following electronic configurations are not possible? Give reasons.
1. 2, 8, 4
2. 3, 8, 2
3. 2, 8, 9
Answer: 2. 3, 8, 2 3. 2, 8, 9
Because according to Bohr Buryscheme, the first shell can have maximum2 and the outermost shell can have a maximumof8 electrons only
4. Why are anode rays called canal rays?
Answer: The anode rays produced at the anode of the discharge tube are called canal rays because they pass through the holes ofthe cathode.
5. What observation scattering experiment prove that the most of the space in the atom is empty?
Answer: most of the Alpha particles pass through the thin foil of gold without undergoing any deflection.
6. Represent the three isotopes of Hydrogen.
Answer: Protium, Deuterium Tritium
7. What are cations and anions?
Answer: The positively charged species are called cations.
Example Na+
The negatively charged species are called anions.
Example: Cl
Structure Of The Atom Class 9 KSEEB Solutions
8. Write the elec tronic configuration of
1. K – (19)
2. Ca – (20)
Answer:
K = 2, 8, 8, 1
Ca = 2, 8, 8,2
9. What is electronic configuration?
Answer: The distribution of electrons in various energy levels is called electronic configuration.
Answer the following
1. Compare any three important characteristics of isotopes with those of isobars.
2. What information do you get from the given figure about the atomic number, mass number and valency of atoms X,Y, and Z? Give your answer in a tabular form

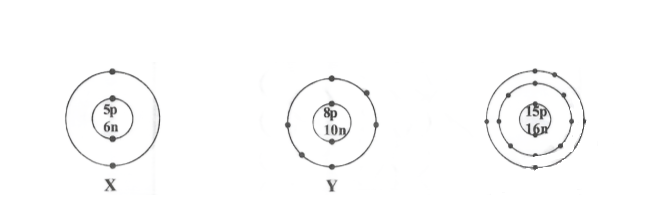
| Element | Atomic No | Mass No ( No of P) | No of Electron (No of P + N) | Electronic Config* | Valency |
| X | 5 | 5+6=11 | 5 | 2,3 | 3 |
| Y | 18 | 8+10-=18 | 8 | 2,6 | 2 |
| Z | 15 | 15+16=31 | 15 | 2,8,5 | 3,5 |
3. List the differences between electron, proton, and neutron
| Electron | Proton | Neutron |
| It has a negative charge | It has a positive charge | It has no charge |
| It is present in orbits around the nucleus of atom | It is present in nuclues of atom | It is present in nucleus of atom |
4. List the properties of cathode rays.
Answer:
1. Cathode rays travel in straight lines.
2. Cathode rays are made up of material particles.
3. Cathode rays produce heating effect.
4. Cathode rays carry negative charge.
5. Cathode rays produce X-rays when they strike against the surface of hard metals like tungsten.
KSEEB Class 9 Science Chapter 4 Exercise Solutions
5. List the properties of anode rays.
Answer:
1. Anode rays travel in straight lines.
2. Anode rays are made up of material particles.
3. Anode rays carry positive charge.
4. Mass of the positively charged particles constituting the anode rays also depend upon the nature of the gas.
1. Rutherford’s model of an atom
Answer:
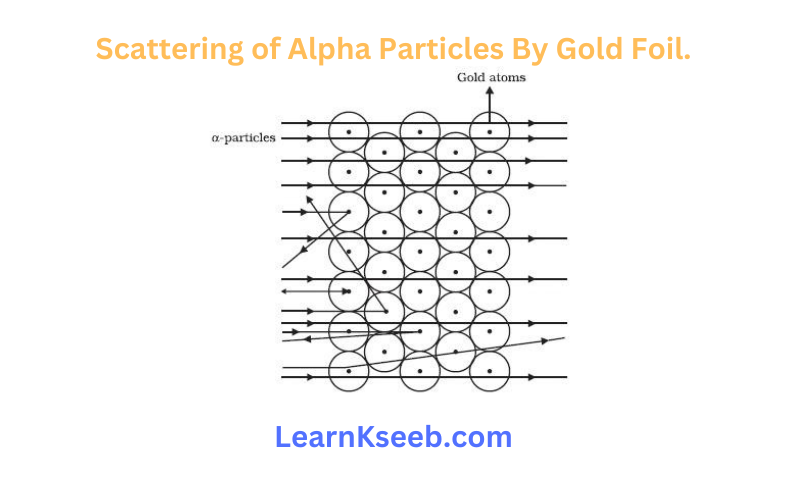
Experiment
Scattering of alpha particles by gold foil.
Observations
1. Most of the Alpha particles passed straight through the gold foil.
2. Some of the Alpha particles were deflected by the foil by small angles.
3. One out of every 12000 particles appeared to rebound.
Conclusions
1. Most of the space inside the atom is empty.
2. The positive charge of the atom occupies very little space.
Rutherford’s nuclear model of an atom
1. There is positively charged center in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
2. The electrons revolve around the nucleus in well-defined orbits.
3. The size of the nucleus is very small as compared to the size of the atom
2. Schematic atomic structure of the first18 elements

